Sodium (Na, Natrium) is a substance with which we deal every day. When you add baking soda for fluffiness or add salt to soup for flavor, you don’t even think about the fact that you are thus replenishing sodium reserves in your body.
- Foods that contain sodium
- Why is sodium needed in salt?
- Lack of sodium in the human body
- What are the dangers of excess sodium?
- Daily value of sodium
- Functions of sodium in the human body
Like many other microelements, people began to use this long before its official discovery. So the ancient Egyptians discovered soda in lakes, which they called “natron,” and found a wide range of uses for it in everyday life.
Using this sodium compound, the Egyptians bleached canvases, prepared artistic paints and glazes, cooked food, washed the body, and even embalmed the dead - making them into their world-famous mummies. But not only the inhabitants of Ancient Egypt could boast of such active use of soda. The Arabs, Greeks and Romans of that time also used it for similar purposes.
The term “sodium” itself comes from the Latin word “natrium”, which, in turn, was borrowed from the Egyptian language and meant “caustic soda”, “soda”.
The discovery of pure sodium belongs to the Englishman Humphry Davy, who at the beginning of the 19th century isolated first potassium, and then the substance of interest to us using electrolysis of molten sodium hydroxide.
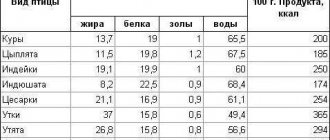
This mineral is found in the form of compounds in sea water and the earth's crust, but we, of course, are interested in more accessible sources of sodium. It's about food. So what foods contain sodium?
Back to contents
How did the name “sodium” come about?
Sodium, Natrium, Na (11)
The name sodium - sodium, natrium comes from an ancient word common in Egypt, among the ancient Greeks (vixpov) and Romans. It is found in Pliny (Nitron) and other ancient authors and corresponds to the Hebrew neter. In ancient Egypt, natron, or nitron, was generally called an alkali obtained not only from natural soda lakes, but also from plant ash. It was used for washing, making glazes, and mummifying corpses. In the Middle Ages, the name nitron (nitron, natron, nataron), as well as boron (baurach), also applied to saltpeter (Nitrum). Arab alchemists called alkali alkali. With the discovery of gunpowder in Europe, saltpeter (Sal Petrae) began to be strictly distinguished from alkalis, and in the 17th century. already distinguished between non-volatile, or fixed alkalis, and volatile alkali (Alkali volatile). At the same time, a difference was established between vegetable (Alkali fixum vegetabile - potash) and mineral alkali (Alkali fixum minerale - soda).
At the end of the 18th century. Klaproth introduced the name Natron, or soda, for the mineral alkali, and for the vegetable alkali, Kali. Lavoisier did not place alkalis in the “Table of Simple Bodies,” indicating in a note to it that these were probably complex substances that once Someday they will be decomposed. Indeed, in 1807 Davy, by electrolysis of slightly moistened solid alkalis, obtained free metals - potassium and sodium, calling them potassium and sodium. The following year, Gilbert, publisher of the famous Annals of Physics, proposed calling the new metals potassium and sodium (Natronium); Berzelius shortened the latter name to “sodium” (Natrium). At the beginning of the 19th century. in Russia sodium was called sodia (Dvigubsky, 182i; Solovyov, 1824); Strakhov proposed the name sod (1825). Sodium salts were called, for example, soda sulfate, hydrochloric soda, and at the same time acetic soda (Dvigubsky, 1828). Hess, following the example of Berzelius, introduced the name sodium.
Foods you should avoid
Some foods contain very high amounts of salt, and it is advisable to limit their consumption. These include:
- all sausages, boiled pork, ham;
- pickled, canned vegetables and pickles;
- sauces – mayonnaise, ketchup, soy vinegar;
- cheese;
- salted and dried fish;
- crackers and nuts with salt, chips;
- baking soda and confectionery products containing it.

History and origin of the name
Sodium (or rather, its compounds) has been used since ancient times. For example, soda (natron), found naturally in the waters of soda lakes in Egypt. The ancient Egyptians used natural soda for embalming, bleaching canvas, cooking food, and making paints and glazes. Pliny the Elder writes that in the Nile Delta, soda (it contained a sufficient proportion of impurities) was isolated from river water. It went on sale in the form of large pieces, colored gray or even black due to the admixture of coal.
Sodium was first obtained by the English chemist Humphry Davy in 1807 by electrolysis of solid NaOH.
The name "sodium" (natrium) comes from the Arabic natrun in Greek - nitron and originally it referred to natural soda. The element itself was previously called Sodium.
Editorial conclusion.
It seems that we can expect new research in this area in the near future.
Not so long ago, fats were the main problem of humanity, but now they “shout” that fats are “good”, but carbohydrates are “evil”. It’s just that someone needs to conduct research, someone needs to promote themselves, someone else needs to make money from it. That's all.
In fact, there were no significant changes. The main harm in fats still comes from trans fats. Fats themselves, like carbohydrates and proteins, as well as all minerals, are necessary for our body.
Once again, we want to convey to our readers that everything should be in moderation. Don't overeat and watch what you eat.
, for today 1
Receipt
The first method of producing sodium was the reduction reaction of sodium carbonate
coal when heating a close mixture of these substances in an iron container to 1000°C:
Na2CO3+2C=2Na+3CO
Then another method of producing sodium appeared - electrolysis of molten sodium hydroxide or sodium chloride.
Receiving technologies
- Ruthenium is extracted from platinum or nickel-copper ore. Moreover, they do this when the raw materials are “purified” of other valuable elements. That is, the main source of metal is the waste remaining as a result of the purification of platinum or metals of its group. The full cycle (including ore enrichment and thermochemical effects) takes one and a half months.
- The second source is the contents of spent fuel rods, nuclear waste. The materials are uranium, thorium, and plutonium. A ton of radioactive decay product contains up to 250 grams of ruthenium.
- The third is a technetium isotope irradiated by a neutron flux.
Minerals, deposits
Minerals found in nature:
- mirabilite (Glauber's salt);
- halite (rock salt);
- borax (formula Na2B4O7 • 10H2O);
- cryolite.

There are no deposits of metallic sodium on the planet. In any place (even in deserts) there is water with which the metal will instantly react.
Extraction and processing
To produce sodium, minerals are mined in many countries:
- Russia;
- USA;
- Germany;
- Mexico;
- Italy.
Industrial production of sodium using the Deville method, common in the 19th century. AC - iron tube with a mixture of soda, coal and chalk; B - Donny and Maresca refrigerator; R - receiver with oil
The main method of industrial production of sodium is electrolysis of NaOH or NaCl.
It can be obtained by thermal decomposition of NaN3.

Qualitative determination of sodium using a flame - bright yellow color of the emission spectrum “sodium D-line”, spectral doublet 588.9950 and 589.5924 nm
Chemical properties
An alkali metal that oxidizes easily in air. To protect against atmospheric oxygen, sodium metal is stored under a layer of kerosene.
.
Sodium is less active than lithium
, therefore it reacts with
nitrogen
only when heated:
2Na + 3N2=2NaN3
When there is a large excess of oxygen, sodium peroxide is formed
2Na + O2 = Na2O2
Oxidation and combustion
Now we can move on to the chemical properties of sodium (Na). This alkali metal, when exposed to air, easily oxidizes. As a result, sodium oxide (Na2O) is formed. It looks like colorless cubic crystals. This is a salt-forming binary inorganic substance that is used as a reagent in the synthesis process. It is used to make sodium hydroxide and other compounds.
Therefore, to protect the metal from oxygen exposure, it is stored in kerosene.
But during combustion, sodium peroxide (Na2O2) is formed. They look like white-yellow crystals, which are characterized by vigorous interaction with water, accompanied by the release of heat. Na2O2 is used for bleaching silk, wool, fabrics, straw, viscose and wood pulp.
Reactions with water
The silvery-white soft metal sodium also interacts successfully with H2O. The reaction with water is very violent. A small piece of sodium placed in this liquid floats to the surface and begins to melt due to the heat generated. As a result, it turns into a white ball, which moves quickly along the surface of the water in different directions.
This very spectacular reaction is accompanied by the release of hydrogen. When conducting such an experiment, care must be taken as it may ignite. And everything happens according to the following equation: 2Na + 2H2O → 2NaOH + H2↑.

Interactions with nonmetals
Sodium is a metal, it can also be called a strong reducing agent, which it is. Like other alkaline substances, however. So it reacts vigorously with many nonmetals other than carbon, iodine, and noble gases, which include radioactive radon, krypton, neon, xenon, argon, and helium. Such reactions look like this: 2Na + Cl2 → 2NaCl. Or here’s another example: 2Na + H2 → 250-450 °C 2NaH.
It is worth noting that sodium is more active than lithium. In principle, it can react with nitrogen, but very poorly (in a glow discharge). As a result of this interaction, an unstable substance called sodium nitride is formed. These are dark gray crystals that react with water and decompose when heated. They are formed according to the equation: 6Na + N2 → 2Na3N.

Reactions with acids
They should also be listed, talking about the chemical characteristics of sodium. This substance reacts with dilute acids like an ordinary metal. It looks like this: 2Na + 2HCl → 2NaCl + H2↑.
Sodium interacts differently with concentrated substances that are characterized by oxidative reactions; such reactions are accompanied by the release of reduction products. Here is an example of a formula: 8Na + 10NHO3 → 8NaNO3 + 3H2O.
It is also worth noting that the alkali metal sodium easily dissolves in liquid ammonia (NH3), a 10% solution of which is well known to everyone as ammonia. The equation looks like this: Na + 4NH3 → -40°C Na [NH3]4. As a result of this reaction, a blue solution is formed.
The metal also interacts with gaseous ammonia, but when heated. This reaction looks like this: 2Na + 2NH3 → 350°C2NaNH2 + H2.

Other connections
When listing the main properties of sodium, it is also worth mentioning that it can interact with mercury, a unique element that under normal conditions is a white-silver heavy liquid, while being a metal.
As a result of this reaction, an alloy is formed. Its exact name is sodium amalgam. This substance is used as a reducing agent, its properties being softer than pure metal. If you heat it with potassium, you get a liquid alloy.
This metal can also dissolve in so-called crown ethers - macroheterocyclic compounds, but only in the presence of organic solvents. As a result of this reaction, an alkalide (a salt, a strong reducing agent) or an electride (a blue solvent) is formed.
It is also impossible not to mention that alkyl halides, which are halogen-carbon substances, with an excess of sodium give organosodium compounds. In air they usually ignite spontaneously. And in water they explode.

Application
Sodium metal is widely used in preparative chemistry and industry as a strong reducing agent, including in metallurgy. Sodium is used in the production of highly energy-intensive sodium-sulfur batteries. It is also used in truck exhaust valves as a heat sink. Occasionally, sodium metal is used as a material for electrical wires intended to carry very high currents.
Alloyed with potassium, as well as rubidium and cesium
used as a highly efficient coolant.
In particular, the alloy of composition sodium 12%, potassium
47%,
cesium
41% has a record low melting point of −78 °C and was proposed as a working fluid for ion rocket engines and a coolant for nuclear power plants.
Sodium is also used in high and low pressure discharge lamps (HPLD and LPLD). NLVD lamps of the DNaT (Arc Sodium Tubular) type are very widely used in street lighting. They give off a bright yellow light. The service life of HPS lamps is 12-24 thousand hours. Therefore, gas-discharge lamps of the HPS type are indispensable for urban, architectural and industrial lighting. There are also lamps DNaS, DNaMT (Arc Sodium Matte), DNaZ (Arc Sodium Mirror) and DNaTBR (Arc Sodium Tubular Without Mercury).
Sodium metal is used in the qualitative analysis of organic matter. The alloy of sodium and the test substance is neutralized with ethanol,
add a few milliliters of distilled water and divide into 3 parts, J. Lassaigne's test (1843), aimed at determining nitrogen, sulfur and halogens (Beilstein test)
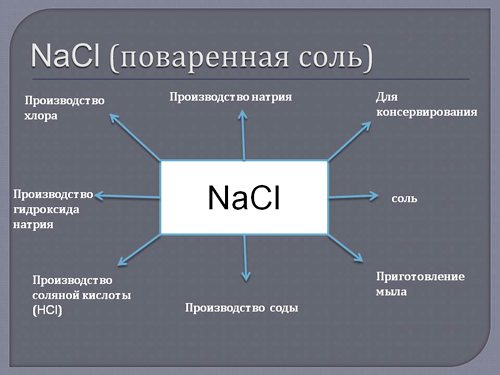
— Sodium chloride (table salt) is the oldest used flavoring and preservative. — Sodium azide (Na3N) is used as a nitriding agent in metallurgy and in the production of lead azide. — Sodium cyanide (NaCN) is used in the hydrometallurgical method of leaching gold from rocks, as well as in the nitrocarburization of steel and in electroplating (silvering, gilding). — Sodium chlorate (NaClO3) is used to destroy unwanted vegetation on railway tracks.
Industry
The main consumer of metal.
The following industries take it:
- Electronics production.
- Electrochemistry.
- Chemical production.
These areas use 90% of raw materials.
Chemists value the metal for its inertness and catalyst properties:
- It is endowed with the unique property of selectivity in reactions. For this it is in demand in the synthesis of organic and inorganic substances.
- When paired with it, the catalytic activity of platinum increases.
- Ruthenium compounds are used as superoxidants, pigments in glass and enamels.
Advanced industries cannot do without ruthenium as an alloy element:
- They are used to make components and contacts of high-precision devices for the needs of radio, electrical, aerospace technology, and the defense complex. For this, 1.1-4.9% ruthenium in the alloy is sufficient.
- 0.1% ruthenium added to titanium multiplies the anti-corrosion properties of the latter.
- Ruthenium-platinum symbiosis is a material for the manufacture of fuel systems and water purification systems on spacecraft.
- Refractory metal is added to alloys in the manufacture of instruments that measure ultra-high temperatures.
Pure ruthenium is used to coat particularly important parts to make them more mechanically and chemically stable.
Jewelry
- In jewelry, the use of metal is twofold: as a reinforcing component of the alloy and as a durable coating .
- Products made from alloys with ruthenium alloy are reliable and durable, which is important for jewelry that experiences increased loads (rings, rings, bracelets).
- Ruthenium coating creates a film on the surface of the product in a range of shades from graphite to black.
Together with rhodium they create elite black gold.
What is interesting for scientists
Ruthenium has cast a spell on many generations of scientists. It turned out that some of its characteristics almost copy the platinoids rhodium and osmium, others - iron.
But almost two hundred years after the discovery of the element, questions remain:
- The main obstacle is created by valence. Ruthenium has eight types. This is not the worst thing. During experiments, valence spontaneously and unexpectedly changes.
- Non-standard catalytic capabilities. It is difficult to vouch for the outcome of the process: the metal itself “decides” which element to help accelerate and which not.
Therefore, it has not yet been possible to obtain stable pure metal.
These circumstances only provoke scientists, especially young people. They intend to tame the wayward metal. Particularly attractive areas are the complete and economically optimal extraction of the element from minerals, sludge, and nuclear waste.
Like platinum, nanodoses of ruthenium are present in the tissues of biological organisms (in humans, these are muscles).
The metal and its compounds are biologically active. This property is used by dermatologists, oncologists, and phthisiatricians.
Of particular note is the inorganic dye known as “ruthenium red.” It contrasts tissue and other biological substances examined under a microscope.
However, there are compounds that are dangerous to humans:
- Dioxide Very poisonous, causes vomiting and suffocation. Provokes allergies, ulcers on the mucous membranes.
- Quadrivalent oxide . Exposure to organics (for example, alcohol) or heating above 100°C is enough to cause an explosion.
This should be taken into account if there are fans of home chemical experiments in the family. Such substances are stored in special containers.
Biological significance of sodium chloride or salt
A crystal of table salt, which has an ionic chemical bond, is necessary for the full life and activity of humans and other living organisms. Sodium chloride takes part in regulating and maintaining water-salt balance and alkaline metabolism. Biological mechanisms control the constant concentration of sodium chloride in various fluids, such as blood.
The difference in NaCl concentrations inside and outside the cell is the main mechanism for the entry of nutrients into the cell, as well as the removal of waste products. A similar process is used in the generation and transmission of impulses by neurons. Also, the chlorine anion in this compound is the main material for the formation of hydrochloric acid, the most important component of gastric juice.
The daily requirement for this substance is from 1.5 to 4 grams, and for hot climates the dose of sodium chloride increases several times.
The body does not need the compound itself, but the Na+ cation and the Cl- anion. If the amount of these ions is insufficient, muscle and bone tissue is destroyed. Depression, mental and nervous diseases, disturbances in the cardiovascular system and digestive processes, muscle spasms, anorexia, and osteoporosis appear.
Chronic lack of Na+ and Cl- ions leads to death. Biochemist Zhores Medvedev noted that with the complete absence of salt in the body, one can last no more than 11 days.
Even in ancient times, tribes of cattle breeders and hunters consumed raw meat products to satisfy the body's need for salt. Agricultural tribes consumed plant foods that contained small amounts of sodium chloride. Signs indicating a lack of salt include weakness and headache, nausea, and dizziness.

Production Features
In the distant past, salt was extracted by burning certain plants in fires. The resulting ash was used as a seasoning.
Table salt obtained by evaporating sea water was not purified; the resulting substance was immediately consumed as food. This technology originated in countries with hot and dry climates, where a similar process occurred without human intervention, and then, when other countries adopted it, sea water began to be heated artificially.
Saltworks were built on the shores of the White Sea, in which concentrated brine and fresh water were obtained by evaporation and freezing.

Natural deposits
Among the places characterized by large reserves of table salt, we highlight:
- Artemovskoye field, located in the Donetsk region. Salt is extracted here using the mine method;
- Lake Baskunchak, transportation is carried out along a specially built railway;
- potassium salts were found in large quantities in the Verkhnekamskoye deposit, where this mineral is mined using the mine method;
- mining was carried out in the Odessa estuaries until 1931; currently the deposit is not used on an industrial scale;
- In the Seregovskoye deposit, brine is evaporated.
Salt mine
The biological properties of table salt made it an important economic object. As of 2006, about 4.5 million tons of this mineral were used on the Russian market, with 0.56 million tons going for food consumption, and the remaining 4 million tons going to the needs of the chemical industry.

physical characteristics
Let's look at some properties of table salt. This substance dissolves quite well in water, and the process is influenced by several factors:
- temperature;
- presence of impurities.
A crystal of table salt contains impurities in the form of calcium and magnesium cations. This is why sodium chloride absorbs water (it becomes damp in the air). If such ions are not part of table salt, this property is absent.
The melting point of table salt is 800.8 °C, which indicates the strong crystalline structure of this compound. Mixing fine sodium chloride powder with crushed ice produces a high quality coolant.
For example, 100 g of ice and 30 g of table salt can reduce the temperature to −20 °C. The reason for this phenomenon is that the table salt solution freezes at temperatures below 0 °C. Ice, for which this value is the melting point, melts in such a solution, absorbing heat from the environment.
The high melting point of table salt explains its thermodynamic characteristics, as well as its high dielectric constant - 6.3.

Receipt
Considering how important the biological and chemical properties of table salt and its significant natural reserves are, there is no need to develop an option for the industrial production of this substance. Let's look at laboratory options for producing sodium chloride:
- This compound can be obtained as a product by reacting copper (2) sulfate with barium chloride. After removing the precipitate, which is barium sulfate, and evaporating the filtrate, crystals of table salt can be obtained.
- When sodium exothermicly combines with chlorine gas, sodium chloride is also formed, and the process is accompanied by the release of a significant amount of heat (exothermic form).
Recommendations for reducing salt intake
- Adults. WHO recommends that adults consume less than 5 g of salt per day (just under one teaspoon) (1).
- Children. For children aged two to 15 years, WHO recommends that the recommended maximum salt intake be adjusted downward based on their energy needs compared to adults. This recommendation for children does not cover the period of exclusive breastfeeding (0–6 months) or the period of complementary feeding while continuing breastfeeding (6–24 months).
- All salt consumed must be iodized, i.e. fortified with iodine, which is essential for healthy brain development in fetuses and young children and overall mental health in all people.
Biological role
In the body, sodium is found mostly outside the cells (about 15 times more than in the cytoplasm). This difference is maintained by the sodium-potassium pump, which pumps out sodium trapped inside the cell.
Together with
potassium , sodium performs the following functions:
- Creating conditions for the occurrence of membrane potential and muscle contractions.
- Maintaining blood osmotic concentration.
- Maintaining acid-base balance.
- Normalization of water balance.
- Ensuring membrane transport.
- Activation of many enzymes.
The recommended dose of sodium is 600 to 1700 milligrams for children, and 1200 to 2300 milligrams for adults. In the form of table salt, this amounts to 3 to 6 grams per day.
Sodium is found in almost all foods, although the body gets most of it from table salt. Absorption mainly occurs in the stomach and small intestine. Vitamin D improves the absorption of sodium, however, excessively salty foods and foods rich in protein interfere with normal absorption. The amount of sodium taken in from food shows the sodium content in the urine. Sodium-rich foods are characterized by accelerated excretion.
Sodium deficiency in a person eating a balanced diet
does not occur in humans, however, some problems can arise with vegetarian diets.
Temporary deficiency may be caused by diuretic use, diarrhea, excessive sweating, or excess water intake. Symptoms of sodium deficiency include weight loss, vomiting, gas formation in the gastrointestinal tract, and impaired absorption of amino acids and monosaccharides
. Long-term deficiency causes muscle cramps and neuralgia.
Excess sodium causes swelling of the legs and face, as well as increased excretion of potassium in the urine. The maximum amount of salt that can be processed by the kidneys is approximately 20-30 grams; any larger amount is life-threatening.
Beneficial properties of sodium and its effect on the body
The beneficial properties of sodium for the body are:
- Normalization of water-salt metabolism;
- Activation of enzymes of the salivary and pancreas;
- Participation in the production of gastric juice;
- Maintaining normal acid-base balance;
- Generating functions of the nervous and muscular system;
- Vasodilator effect;
- Maintaining blood osmotic concentration.
Sodium digestibility
Sodium is found in almost all foods, although the body gets most of it (about 80%) from table salt. Absorption mainly occurs in the stomach and small intestine. Vitamin D improves the absorption of sodium, however, excessively salty foods and foods rich in protein interfere with normal absorption.
Interaction with others
Increased sodium intake causes fluid accumulation in the body, swelling, and increases blood pressure. Large sodium (salt) intake will deplete potassium, calcium and magnesium.
Uses of sodium in life
The use of sodium metal is in the chemical and metallurgical industries, where it acts as a powerful reducing agent. Sodium chloride (table salt) is used by all inhabitants of our planet without exception; it is the most famous flavoring agent and the oldest preservative.
Signs of sodium deficiency
Sodium deficiency usually occurs when you sweat excessively, such as in hot climates or during exercise. A lack of sodium in the body is characterized by memory impairment and loss of appetite, dizziness, fatigue, dehydration, muscle weakness, and sometimes cramps, skin rashes, stomach cramps, nausea, and vomiting.
Conclusion from the study
The researchers concluded that everyone benefits from reducing their sodium intake. However, many questions remain; critics argue that the experiment was very short-term in order to refute generally accepted recommendations. They hastened to remind us of the harm caused by a lack of the mineral in the blood. Supporters of the study argue that most people do not need more than 1500 mg for normal functioning and health. Due to the short duration of the experiment, it was not possible to establish the benefits of this diet for preventing the risk of cardiovascular diseases.
A group of scientists led by Harvard researchers found participants in experiments on the prevention of hypertension (HTP), conducted in 1980 and 1990. This can already be called long-term experiments. It was found that people who reduced their sodium intake to 2000-2600 mg per day had almost 30% fewer cases of cardiovascular diseases and reduced mortality.
The debate over whether salt restriction is right for everyone will likely continue, as will the debate on virtually all health topics.
The effect salt has on a person's blood pressure and health depends on many things, including:
- Genetic predisposition
- Person's age
- Lifestyle
- Human physical health.