Proteins are substances of organic origin. Proteins are the most essential elements for living beings. Non-periodic polymers, which include proteins, are different from other polymers.
The first protein was isolated in the form of gluten in 1728 by the Italian Jacopo Bartolomeo Beccari, who lived in 1682-1766, from wheat flour. Washed gluten is a complex of water-insoluble glutens, which includes proteins. From this moment the study of protein began.
The structure of protein molecules
Their molecules are built from similar but different monomers - 20 amino acids. Each of them has its own name, structure and properties. Its molecule is built from a specific radical and a component common to all, which includes an amino group with alkaline properties and a carboxyl group with acidic properties. The presence of both groups in one molecule determines their high degree of reactivity.
These groups connect high-molecular nitrogen-containing organic substances, forming new polymers, which include proteins. The process occurs as follows: from the amino group of one amino acid and the carboxyl of another, a water molecule is formed, and free electrons are bonded to form a peptide bond. This is how polypeptides are formed. All that the human body can do with the help of cells is the action of proteins.
Amino acid composition
Amino acids, as their name suggests, contain a basic amino group (-NH2) as well as an acidic carboxyl group (-COOH), both bonded to a central carbon atom. The carbon is additionally bonded to a hydrogen and a functional protein group called a radical (R). These components completely fill all the bonds of the central carbon atom.
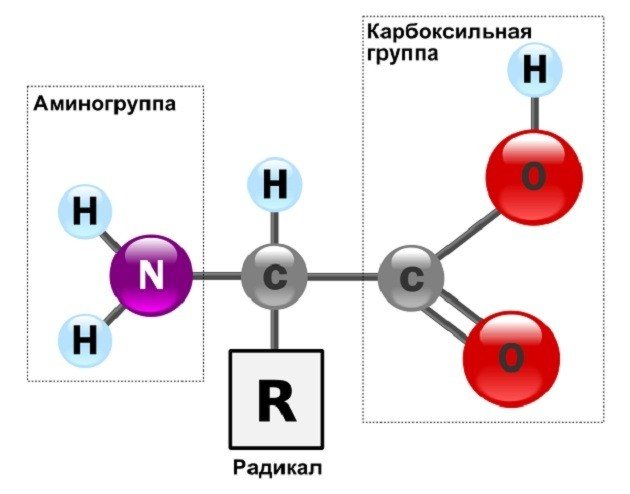
General structure of α-amino acids that make up proteins (except proline). Author: User:X-romix
The unique character of each amino acid is determined by the nature of the radical group. Please note that if the radical group does not contain a hydrogen atom (H), as in glycine, then the amino acid is chiral and can exist in the form of two enantiomers: d or L. Proteins in living systems usually contain α (L)-amino acids, and β ( d)-amino acids are extremely rare.
The radical group determines the chemical properties of amino acids - they can be polar or non-polar, hydrophobic or hydrophilic. Serine with the radical -CH2OH is a polar molecule, Alanine, which has –CH3 as a radical group, is non-polar.
There are also basic amino acids (with more than one amino group) and acidic amino acids (with more than one carboxyl group). The presence of an additional amino or carboxyl group affects the properties of the amino acid, which play a decisive role in the formation of the spatial structure of the protein.
The radical of some amino acids (for example, cysteine) contains sulfur atoms. All 20 amino acids are grouped into five chemical classes based on their radical group.
- Non-polar amino acids such as leucine often have -CH2 or -CH3 as their radical.
- Polar uncharged amino acids, such as threonine, with a radical containing an oxygen or hydroxyl group (-OH).
- Charged amino acids, such as glutamic acid, with a radical having acids or bases capable of ionization.
- Aromatic amino acids such as phenylalanine, which has a radical group containing an organic (carbon) ring with alternating single and double bonds. They are also non-polar.
- Amino acids with special functions and properties. For example, methionine, which is often the first amino acid in a protein chain, proline, which causes kinks in the chains, cystine, which links the chains together.
Each amino acid affects the shape of the protein differently, depending on the chemical nature of the side groups. For example, parts of a protein chain with numerous nonpolar amino acids fold within their own chain by hydrophobic exclusion.
Biosynthesis in pharmacy
Knowledge of the processes of protein biosynthesis is of great importance for health protection, in particular, for pharmacy, it provides an explanation for the occurrence of genetic diseases, and solves the problem of their prevention and treatment. Used for the synthesis of pharmaceuticals, including antimetabolites. These drugs are used to stop protein biosynthesis processes in oncology: antimutagens that protect DNA from mutations; radioprotectors that protect nucleic acids from radiation changes.
Protein biosynthesis helps to reveal the principles of the effects of medications, such as antibiotics, which inhibit protein biosynthesis in microorganisms and viruses at various stages.
What about proteins?
Protein, or protein, can be of animal or plant origin. What belongs to which proteins? A huge source of protein is green plants, soy products, grains, nuts and beans. When an animal eats plant food, the plant protein is converted into animal protein in its body. Therefore, animal protein is found in meat, milk, and raw eggs. A person has the choice to consume protein-rich plant or animal foods.

Digestibility of various proteins
Of course, proteins in the same group differ in their properties. Depending on which proteins the product belongs to, it has different digestibility. Some are quickly absorbed and speed up metabolism, while others can simply pollute the body. Let's look at what substances belong to proteins.
The leader among proteins that are quickly digested is the egg. It should be eaten after training, because the egg does not leave behind a layer of fat like, for example, other animal proteins.
Fish is an excellent source of protein, it is easily digestible and does not cause heaviness in the stomach. As for animal meat, it is the undoubted leader in the amount of protein per 100 g. And if you eat 250 g of meat, then our body will receive the daily requirement of protein. But poultry meat compromises somewhat on its protein content, but it is lean and easier to digest.
As for plant proteins, the leader is soybean, which contains almost the same protein content as an egg. You should add nuts and seeds to your diet, the protein percentage of which reaches the same level as poultry meat, but due to their high calorie content they need to be consumed little by little.
The effect of protein on human satiety control
Scientists have noticed that some animals begin to behave differently due to fluctuations in protein in their diet. This is the so-called protein lever hypothesis. Its meaning is that our smaller brothers first try to fill the body with protein, and only then load up on carbohydrates and fats. Thus, they control overeating as protein reduces hunger.
Macronutrient fluctuations were described using a mathematical model. She proved that different people around the world consume protein within 10-15%, but the proportions of fat and carbohydrates are very different. Our hunter-gatherer ancestors ate high-protein foods, which made up 20-30% of their total diet.
Many people believe that to lose weight and maintain weight, you just need to increase your protein intake. However, scientists have conducted a number of studies and found that the diet of overweight people contains almost the same amount of protein as thin people. Thus, weight loss is influenced not so much by increasing protein, but by reducing the amount of foods with a high glycemic index. In addition, it has been proven that long-term protein diets lead to health problems.
At the same time, the positive effect of proteins on the human body cannot be denied. Protein perfectly saturates the body and helps control hunger. How to use this property of protein leverage wisely?
- The right food. It is better to give preference to whole foods containing protein and not to abuse their combinations with fats and carbohydrates (for example, eating potatoes and meat together). Because in this case, in order to gain the protein norm, you will have to eat too many carbohydrates and fats “for company.”
- Protein for breakfast. This method is guaranteed to reduce appetite during the day by 51%. The first meal with a high protein content is justified and even recommended for everyone who is concerned about and monitors their figure and body quality in general.
- It is recommended to start any meal with protein. I immediately remember the ancient expression “Ab ovo”, which translated means “let's start with an egg.” Protein at the beginning of your meal, like a vegetable salad, helps reduce the number of calories you consume.
- On days when you have a strong appetite, eat plenty of protein. And during periods of rest, reduce its amount by taking a break.
Use of proteins, carbohydrates and fats in the diet
If we approach nutrition from a scientific point of view, then a person does not require specific food products, but their elements for the growth and functioning of the body. These are 3 groups of substances: proteins, fats, carbohydrates. All of them must be present in a specific amount every day, otherwise disruptions in the body and deterioration in health are possible. There is also a certain ratio between these substances that must be adhered to.
Nutritionists recommend building your diet taking into account the following norm: 15% proteins, 20% fats and 65% carbohydrates. Of course, this is an average proportion: it depends on the specific organism, height parameters, weight and age of the person. Therefore, subjective numbers should be determined by contacting a nutritionist or trainer.
Biological significance
B. perform numerous. functions. Metabolism (digestion, respiration, excretion, etc.) and the life of the cell as a whole are inextricably linked with the activity of enzymes - highly specific biochemical catalysts. reactions that are B. The protective systems of higher organisms are formed by protective B., which include immunoglobulins, B. complement, cytokines of the immune system, B. of the blood coagulation system (including plasmin, thrombin, fibrin). An important group consists of regulatory bacteria that control the biosynthesis of bacteria and nucleic acids. These also include peptide-protein hormones. Information about the state of the external environment, decomp. regulatory signals are perceived by the cell with the help of receptor proteins located on the outer surface of the plasma cells. membranes. B. signaling systems ($\ce{G}$-proteins, B.-effectors) are responsible for the transmission of signals into the cell, which play an important role in the transmission of nervous excitation and in the oriented movement of the cell (chemotaxis). In the active transport of ions, lipids, sugars and amino acids through biological. membranes are involved in transport B., or B. carriers. Among them, for example, hemoglobin and myoglobin, which transport oxygen. The bone base will connect. tissues, wool, and horny formations make up structural B. (including collagen, keratin, elastin). They also form the cellular skeleton and intercellular matrix. The divergence of chromosomes during cell division, the movement of flagella, and the work of the muscles of animals and humans are carried out according to a single mechanism through the B. contractile system (actin, myosin, tropomyosin, tubulin, etc.). B. is the most important component of human food and animal feed. Of particular importance in this case are the reserve proteins of plants and animals (for example, casein, prolamins). The transformation and utilization of energy entering the body with food, as well as the energy of solar radiation, occurs with the participation of the bioenergy system (for example, rhodopsin, cytochromes). Among the peptide-protein substances there are antibiotics (including gramicidins, polymyxins, enniatins), toxins and other biologically active substances.
The work of proteins
High-molecular biological compounds, which include proteins, are broken down into amino acids, which penetrate into the blood and saturate the body, and it uses them to build its proteins. In order for the body to acquire strong muscles and a beautiful, toned appearance, it is necessary to replenish the diet with proteins. There are enough of them in fish and meat, eggs and cheese, legumes and soy products. The diet should be calculated based on the daily intake of all ingredients, as well as the goals set.

Those who want to pump up their muscles should increase their protein intake, and those who are losing weight should reduce their intake of high-carbohydrate and fatty foods. Proteins, fats, carbohydrates are the main sources of energy and strength. On average, for a healthy diet and maintaining a normal weight, a person needs to take proteins, fats, and carbohydrates with food in the following proportion: 90-110/90-100/250-300 g. For children, these numbers are slightly lower.
Protein foods and daily servings:
- chicken, turkey, lean ham - 75 g;
- pork, lamb, beef - 45 g;
- fatty fish – 30 g; lean fish, seafood – 60 g;
- beans, lentils – 2 tbsp. l.;
- bacon - 1 lean slice;
- fish sticks – 2 pcs.;
- low fat hummus – 1 tbsp. l.;
- eggs - 1 pc.;
- nuts - 15 g.
Muscles are built from protein, and human food must contain it, because it is a source of strength. Proteins from fish and poultry are best absorbed. Products such as legumes, cereals, and soy also contain proteins, and, moreover, a small amount of fat; cheeses and dairy products are not far behind them.
Harm from protein foods
The benefits of protein foods do not negate their dangers. After all, most of these products (especially animals) are considered “heavy” for the body. Restrictions apply to people with the following health problems:
- liver failure;
- Gastrointestinal problems: stomach ulcers, gastritis, dysbacteriosis.
This does not mean a complete rejection of such food, but an obsession with protein diets. Before starting them, consult your doctor.
It is not contraindicated for healthy people, but not more than four weeks. The dominance of proteins at the expense of fats or carbohydrates leads to an imbalance. This is fraught with disruptions in metabolism and the functioning of the genitourinary system. Hair and nails will become brittle and the skin will become dry. And a fan of protein foods makes him nervous, irritable, and anxious.
Protein diet
A fashionable and effective diet, however, contains pitfalls. When deciding to lose weight or make your muscles beautiful by using such products, consider the following:
- Not every food or product rich in polypeptides is healthy. To feel full without filling your body with heavy food, choose low-fat, low-calorie options.
- Processed meat is excluded: sausage, frankfurters, pates. These products are stuffed with “taste improvers”, flavorings, preservatives, and fats. No mayonnaise, sauces, or cheese masses are needed. Their composition negates the usefulness of natural nutrients.
- To preserve the maximum amount of proteins and other useful components of meat, it is boiled, stewed, baked or steamed.
- It has been calculated how much protein the body can absorb in one meal - 30-35 g. Therefore, there is no need to “translate” foods. It is better to eat not three times a day, but five to six times.
- The main (protein) food is supplemented with vegetables, fruits, cereals, and dairy products. This combination guarantees the health of the body.
- At night, an hour and a half before bedtime, let's take a glass of low-fat yogurt or kefir.
- Proteins are the number one source for building muscle tissue. Therefore, the diet should be supplemented with physical activity or active recreation. A wasp waist, a beautiful butt, and firm breasts will not be fantastic.
- It is recommended to use a protein diet no more than once every six months. In case of health problems, the need, duration and frequency are determined by the attending physician.
- Suitable diet for a protein diet: eggs, low-fat dairy products, soy cheese (tofu), meat, salmon fillet, all legumes, whole grain bread.
It is difficult to maintain the purity of the experiment for three or four weeks.
To avoid being completely depressing, the protein diet can be diluted a couple of times with foods such as nuts, baked or boiled potatoes, and medium-fat cottage cheese. However, lard, buns, sweets, French fries, and other similar fats or carbohydrates are expressly prohibited.
A special article is protein powder (shake). This is a protein product that excludes the presence of fat. Absorbed instantly, helps build muscles, makes your figure sculpted. Useful if the diet is complemented by exercise.
Interesting facts about proteins
More than 4 billion years ago on planet Earth, proteins incredibly emerged from insignificant inorganic molecules, which became the origin of life. All living things exist thanks to unique protein molecules, and other forms of life in the Universe are still unknown to scientists.
- Every living organism is made of proteins. They occupy about 50% of the dry matter of any organism. Viruses contain protein from 45 to 95%.
- About 30% of a person's protein comes from muscles, about 20% from bones and tendons, and about 10% from skin.
- There are 1012 different protein substances that give life to organisms of all levels of complexity, from viruses to humans.
- The brain is also a protein. It should be remembered that due to the process of protein denaturation, when ethyl alcohol enters the body, brain cells die.
- Squirrels owe their name to egg white, which has been used by people since ancient times as a component of food.
- The role of proteins in the body is very diverse; they can act as enzymes. Pepsin is an enzyme that breaks down proteins into amino acids in the body.
- Proteins have a protective effect. Interferon is a protein that saves the human body from the invasion of viruses.
- Proteins can play the role of hormones, an example is the hormone insulin. It promotes the entry of glucose into the cell.
- Proteins fulfill the energy task for the body.
- Hair is made of pure protein.
Protein structures
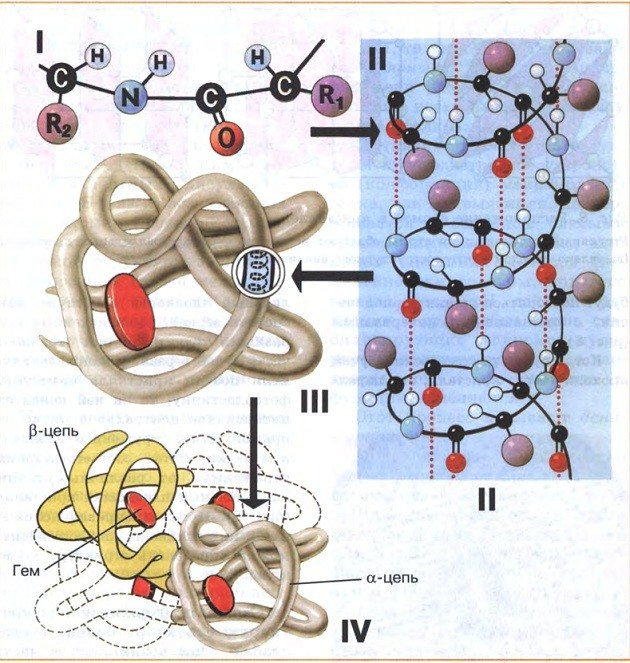
There are 4 spatial organizations of protein molecules:
- primary - created by peptide bonds, it is this that determines the properties of protein molecules;
- the secondary, shaped like an extended spring, is built from peptide and hydrogen bonds (tendons, nails, hair, cobwebs);
- tertiary, spherical in shape, built from peptide, hydrogen and disulfide bonds (enzymes, antibodies, hormones);
- quaternary - consists of alpha and beta chains (hemoglobin).
Each protein is assigned a genetic code, which stores information about what form it should take, but geneticists still cannot predict its spatial structure from the primary code. For a protein to work, it needs to fold in a certain way.
It is very important to know which foods contain protein, what kind it is and how much it should be consumed. Why? But because protein, burning in the body, saturates it with a large amount of energy. It is after high-protein food that we feel a surge of strength; it is protein that builds each of our muscles and creates a beautiful and healthy body. Equally important is the fact that protein helps you lose weight or maintain weight after losing weight.
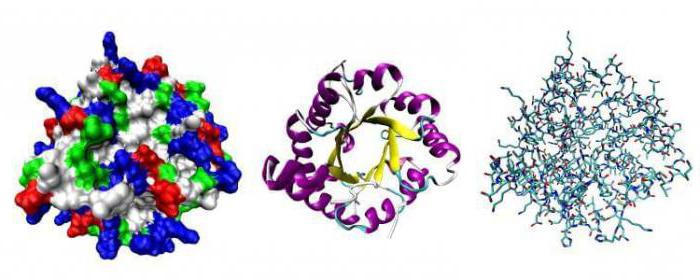
Levels of structural organization of proteins
The shape of a protein determines its function. One way to study something as small as a protein is to look at it using short-wave radiation, which is represented by X-rays. X-rays are passed through the protein to obtain diffraction patterns. This picture is painstakingly analyzed and allows the researcher to construct a three-dimensional image of the molecule with the position of each of its atoms. The first protein analyzed in this way was myoglobin; soon the associated protein hemoglobin was subjected to the same analysis.
When a sufficient number of proteins were studied, a general principle of their structure became apparent: in each protein studied, all internal amino acids, such as leucine, valine and phenylalanine, are non-polar. The tendency of water to exclude non-polar molecules literally pushes such parts of the amino acid chain into the protein. Nonpolar amino acids are forced into close contact with each other, leaving little free space inside the molecule. Polar and charged amino acids are concentrated on the surface of the protein, with the exception of a few that play key functional roles.
The structure of proteins is generally described as a hierarchy of four levels: primary, secondary, tertiary and quaternary. We will consider this view and then integrate it with a more modern approach arising from expanding knowledge of protein structure.
Levels of organization of protein molecules
Primary structure of proteins
The primary structure of a protein is its amino acid sequence, i.e. it is a chain of many amino acid residues connected by peptide bonds. This is the most important structure, as it determines the shape, properties and functions of the protein. Based on the primary structure, other forms of the molecule are created.
The radical groups that distinguish amino acids do not play a role in the peptide chain of proteins, and a protein can include any sequence of amino acids. Since any of the 20 amino acids can appear anywhere, a protein containing 100 monomers can form any of 20,100 different amino acid sequences. This important property of proteins allows them to be diverse, but each of them functions only with a certain amino acid sequence.
Protein secondary structure
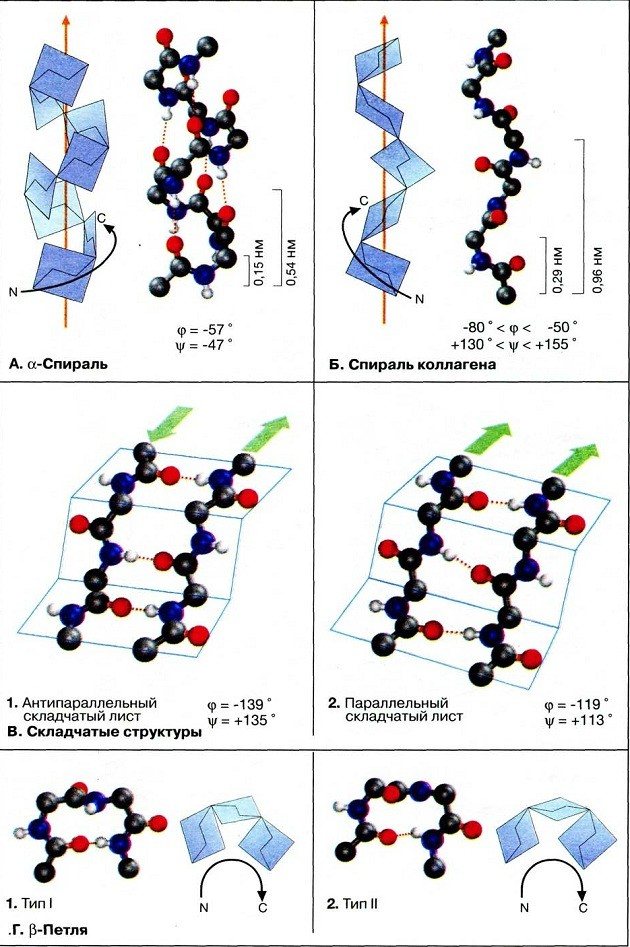
Side and peptide groups of polypeptide chains can form hydrogen bonds. The secondary structure of the protein arises as a result of the bonding of hydrogen atoms of NH groups and oxygen atoms of CO groups. The polypeptide chain becomes helically twisted. Hydrogen bonds are weak, but due to their large number they ensure the stability of this structure. For example, the molecules of keratin, myosin and collagen have a helical configuration.
Hydrogen bonds of peptides can form with water. If there are too many bonds with water, proteins will not be able to acquire a globular structure. Linus Pauling proposed that peptide groups could interact with each other if the peptide was folded into a helix, which he called an α-helix. This type of regular interaction in a peptide forms its secondary structure.
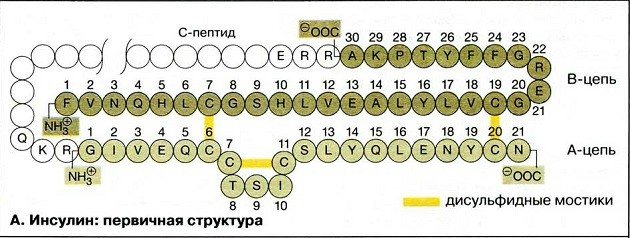
Secondary structure of insulin
Another form of secondary structure is formed between zones of the peptide arranged in a single row, resulting in a flat, folded molecule called a β-sheet. Parts of a protein can be either parallel or antiparallel, depending on whether adjacent sections of the peptide are oriented in the same or opposite directions.
These two types of secondary structure create zones of the protein - cylindrical (α-helices) and flat (β-sheets). The final protein structure may include regions of each type of secondary structure. For example, DNA-binding proteins typically have α-helical regions that can lie across the DNA and interact directly with DNA bases. Porin proteins, which form holes in membranes, are composed of β-sheets. In hemoglobin, α and β structures (globins) have their own zones in the molecule.
Secondary structure of proteins
Tertiary structure of proteins
The final structure of chemically bound proteins is called tertiary. The tertiary structure is formed due to the formation of hydrogen, ionic and other bonds that arise in the aqueous environment between different groups of atoms of the protein molecule of the secondary structure.
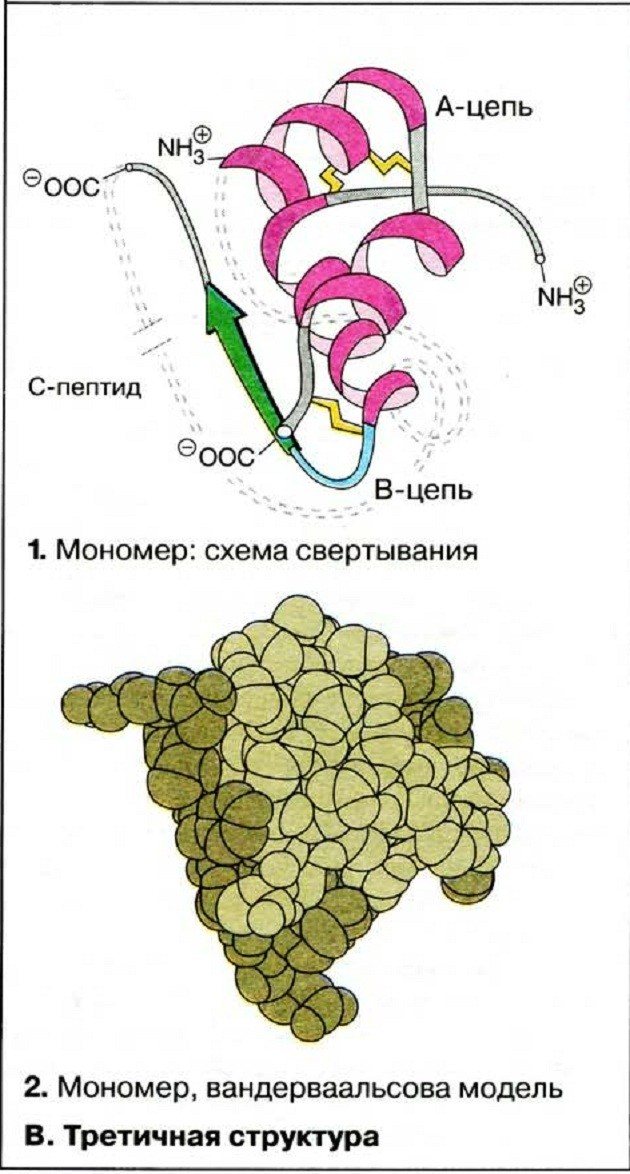
In some proteins, S–S bonds (disulfide) between cysteine residues (an amino acid containing sulfur) play an important role in the formation of the tertiary structure. In this case, the polypeptide helix fits into a kind of ball (globule) in such a way that hydrophobic amino acid radicals are immersed inside the globule, and hydrophilic ones are located on the surface and interact with water molecules. The tertiary structure determines the specificity of protein molecules and their biological activity. Many proteins have it, for example myoglobin (a protein that is involved in creating a supply of oxygen in the muscles) and trypsin (an enzyme that breaks down food proteins in the intestines).
The tertiary structure is stabilized by a number of forces, including:
- hydrogen bonds between radicals of various amino acids;
- electrostatic attraction of radicals with opposite charges;
- hydrophobic exclusion of non-polar radicals;
- covalent disulfide bonds.
At the tertiary structure stage, proteins can be divided into two groups based on the shape of their molecules:
- globular - have a round shape. Blood globulins and albumins, fibrinogen, hemoglobin have this form;
- fibrillar - characterized by an elongated, thread-like shape of molecules. These are keratin, collagen, myosin, elastin, etc.
Quaternary protein structure
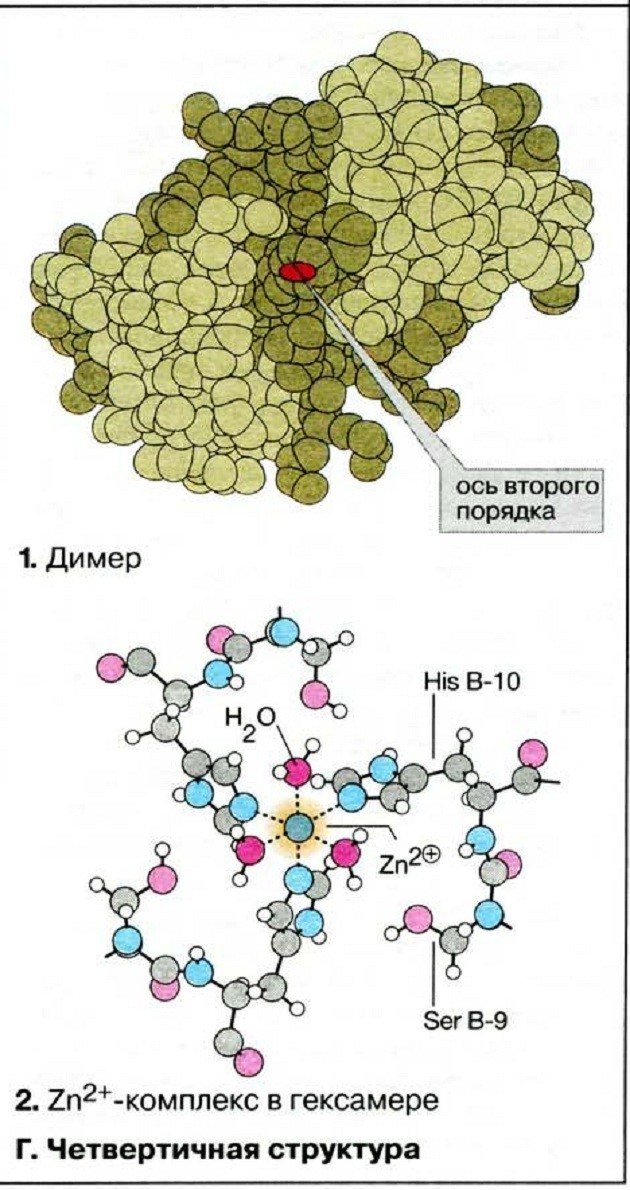
When two or more polypeptides bind to form a functional protein, the individual chains are called subunits. The arrangement of these subunits is the quaternary structure. The subunits in such proteins are most often nonpolar, so they are not chemically bonded and are responsible for separate activities. The strength of the quaternary structure is ensured by the interaction of weak intermolecular forces.
The quaternary structure is characteristic of the hemoglobin protein. Remember that hemoglobin consists of two α-chains and two β-chains, and also includes a non-protein component - heme.
The subunits are arranged in their final quaternary structure. This is the final structure of some, but not all proteins. For proteins that consist of only one polypeptide chain, such as the enzyme lysozyme, the final structure is tertiary.
Motifs and domains - structural elements of proteins
Manually determining the amino acid sequence of a protein is labor-intensive work. This situation was changed by the discovery of the ability of the DNA molecule to store information about a protein. The human genome was originally sequenced by hand. The advent of next-generation technologies has led to a marked acceleration in sequencing.
Today, more than 40,000 bacterial genomes and almost 8,000 eukaryotic genomes have been sequenced, including 80 mammalian gene sequences. Since the composition of DNA is directly related to the sequence of amino acids in proteins, biologists now have a huge database of protein structure.
New information made us think about the logic of the genetic code and the basic laws of protein structure. Researchers still view the four-level hierarchical system as important, but new terms have entered the biologists' vocabulary: folding motif and protein domain.
Protein molecule folding motif
When biologists discovered the tertiary structure of a protein (an even more labor-intensive task than determining the sequence of amino acids in a chain), they noticed similar elements located in dissimilar proteins. Such structures are called motifs, and sometimes “supersecond structures.” The term motif is borrowed from art and refers to a thematic recurring element in music or design.
One common β-α-β motif forms the so-called “Rossmann fold” in a large number of proteins. The second commonly found motif is the β-barrel, which is a β-sheet folded in a circle to form a tube. The third type of motif, helix-turn-helix, consists of two α-helices separated by a bend. It is used by proteins to bind to the DNA molecule.
Researchers still cannot understand the logic of the structure of styling motifs. Perhaps if amino acids are letters in the language of proteins, then motifs are repeated words or phrases. Folding motifs have helped identify unknown protein functions, and the protein motif database is used to search for new unknown proteins.
The folding motifs are quite conserved and occur in proteins that have neither functional nor evolutionary relationships. Determination of folding motifs underlies the physical or rational classification of proteins.
Protein domains
Domains are functional units in the form of a globule within the larger structure of proteins. They can be considered as substructures within the tertiary structure of a protein. In protein language these are “paragraphs”. Most proteins are composed of several domains that perform different parts of the protein's functions.
In many structures these domains can be physically separated. For example, this is how transcription factors are designed - proteins that bind to DNA and initiate the construction of RNA using its complementary DNA. It was found that if the DNA-binding regions are swapped with transcription factors, the specificity of the factor can be changed without changing its ability to stimulate transcription. Domain swapping experiments have been performed with many transcription factors and indicate that the activation and DNA-binding domains act separately.
These formations can also help proteins fold. As the polypeptide chain acquires its structure, the domains take on the correct shape. This effect can be demonstrated experimentally. Artificial production of a polypeptide fragment that forms a domain in an intact protein shows that the fragment folds to form the same structure as the prototype.
Protein related products
It is not for nothing that protein diets are recognized as the most effective among nutritionists, because the body spends a lot of energy on processing protein foods, about 5-10% of incoming calories.
List of the highest protein foods.
- Chicken eggs contain 17% easily digestible protein. Nutritionists advise eating eggs after physical activity, but no more than 2 eggs per day to renew muscle tissue. At the same time, eggs are a low-calorie product.
- Low-fat cottage cheese contains 25% protein and is easily digestible.
- Hard cheese has about 30% protein, but remember that it is too high in calories, and the fatter it is, the less protein it contains.
- Poultry meat is the main source of dietary nutrition, since its calorie content is low and is easily digestible. Poultry meat contains from 15 to 20% protein.
- Beef is low in calories and generous in protein. Just one serving of lean beef contains about 22 grams of protein.
- The liver consists of 25% protein. Boiled and stewed, it is very useful, as it is rich in other essential elements.
- Some types of fish contain up to 25% protein, for example, tuna, salmon, mackerel. Such fish can be used for food without fear of gaining weight.
- The richest plant product in protein is soybeans, which contain 14% protein, while Brussels sprouts are the leading vegetable product (9%).
- Cereals contain at least 10% protein, which is perfectly absorbed by the body.

These are the main products related to proteins. The list given here is far from complete, but takes into account the body's basic protein needs.
Daily protein intake
How much protein a person needs per day depends on gender, body weight, lifestyle, and physical condition of the body. The norm is calculated in grams per kilogram of human body weight. With a sedentary lifestyle (g/kg body weight):
- protein norm per day for women – 1.00;
- for men – 1.20.
Adherents of an active lifestyle, including visiting a sports or gym:

- protein norm for a woman is 1.20;
- men – 1.56-2.00.
Teenagers and pregnant women require 1.20-1.50 grams daily. It is not difficult to calculate how many grams of protein a person needs. For example, a teenager weighing 20 kg needs 24-30 grams daily.