Proteins are substances of organic origin. Proteins are the most essential elements for living beings. Non-periodic polymers, which include proteins, are different from other polymers.
The first protein was isolated in the form of gluten in 1728 by the Italian Jacopo Bartolomeo Beccari, who lived in 1682-1766, from wheat flour. Washed gluten is a complex of water-insoluble glutens, which includes proteins. From this moment the study of protein began.
The structure of protein molecules
Their molecules are built from similar but different monomers - 20 amino acids. Each of them has its own name, structure and properties. Its molecule is built from a specific radical and a component common to all, which includes an amino group with alkaline properties and a carboxyl group with acidic properties. The presence of both groups in one molecule determines their high degree of reactivity.
These groups connect high-molecular nitrogen-containing organic substances, forming new polymers, which include proteins. The process occurs as follows: from the amino group of one amino acid and the carboxyl of another, a water molecule is formed, and free electrons are bonded to form a peptide bond. This is how polypeptides are formed. All that the human body can do with the help of cells is the action of proteins.
Subtleties of structure
When considering the question of what proteins are made of, it is worth studying the structure of this substance. Highlight:
- Primary structure. The basis is the alternation of amino acids in the composition. If at least one element is included or “falls out”, then a new molecule is formed. Thanks to this feature, the total number of the latter reaches an astronomical figure.
- Secondary structure. The peculiarity of the molecules in the protein is that they are not in an extended state, but have different (sometimes complex) configurations. Thanks to this, the life of the cell is simplified. The secondary structure has the form of a spiral formed from uniform turns. In this case, adjacent turns are characterized by close hydrogen bonding. In case of repeated repetition, stability increases.
- The tertiary structure is formed due to the ability of the mentioned spiral to fit into a ball. It is worth knowing that the composition and structure of proteins largely depends on the primary structure. The tertiary base, in turn, guarantees the retention of high-quality bonds between amino acids with different charges.
- Quaternary structure is characteristic of some proteins (hemoglobin). The latter forms not one, but several chains that differ in their primary structure.
The secret of protein molecules is in a general pattern. The higher the structural level, the less well the formed chemical bonds are held together. Thus, secondary, tertiary and quaternary structures are exposed to radiation, high temperatures and other environmental conditions. The result is often a violation of the structure (denaturation). At the same time, a simple protein, if its structure changes, is capable of rapid recovery. If the substance has been subjected to negative temperature action or the influence of other factors, then the denaturation process is irreversible, and the substance itself cannot be restored.
Biosynthesis in pharmacy
Knowledge of the processes of protein biosynthesis is of great importance for health protection, in particular, for pharmacy, it provides an explanation for the occurrence of genetic diseases, and solves the problem of their prevention and treatment. Used for the synthesis of pharmaceuticals, including antimetabolites. These drugs are used to stop protein biosynthesis processes in oncology: antimutagens that protect DNA from mutations; radioprotectors that protect nucleic acids from radiation changes.
Protein biosynthesis helps to reveal the principles of the effects of medications, such as antibiotics, which inhibit protein biosynthesis in microorganisms and viruses at various stages.
Plant-based protein sources for vegetarians
If you do not consume animal products, your main task is to choose a diet that will provide your body with all the necessary components.
You should note that rice, for example, contains too little lysine to be considered a complete source of protein. But by eating it with beans or lentil salad, you'll get all nine essential amino acids.
Which plant foods contain high amounts of protein?
- Quinoa is a gluten-free grain that contains 8 grams of protein per 1 cooked cup (185 grams). The cereal contains many beneficial minerals, including magnesium, iron and zinc.
- Tofu is a cheese made from soy milk. Eating 85g provides approximately 8g of protein. Contains calcium, potassium and iron.
- Buckwheat grain. One cup (168 grams) of cooked buckwheat provides approximately 6 grams of protein. It is a source of many important minerals, including phosphorus, manganese, copper, magnesium and iron.
- Spirulina is a type of blue-green algae; 1 tablespoon (7 g) of dried spirulina provides 4 g of protein. It is rich in antioxidants and is a source of several B vitamins, copper and iron.
- Chia seeds. Two tablespoons (28 g) of seeds provide 4 grams of protein. Is a good source of Omega-3, iron, calcium, magnesium and selenium.
- Rice and beans are a classic combination that provides complete protein. One cup (239 g) of rice and beans provides 12 g of protein and 10 g of fiber.
- Nuts. For example, 30 grams of almonds provides 6 grams of protein, almost the same amount found in 30 grams of grilled ribeye steak.

Plant-based foods high in protein
What about proteins?
Protein, or protein, can be of animal or plant origin. What belongs to which proteins? A huge source of protein is green plants, soy products, grains, nuts and beans. When an animal eats plant food, the plant protein is converted into animal protein in its body. Therefore, animal protein is found in meat, milk, and raw eggs. A person has the choice to consume protein-rich plant or animal foods.

Additional Protein Sources
A healthy source of protein can be called “Isolates”. These are purified proteins of animal and plant origin, in the right ratio, with a high degree of absorption and without extra calories.
The combination of proteins from food and protein shakes in the diet allows you to maintain protein balance, as well as very comfortable and effective weight loss.

Digestibility of various proteins
Of course, proteins in the same group differ in their properties. Depending on which proteins the product belongs to, it has different digestibility. Some are quickly absorbed and speed up metabolism, while others can simply pollute the body. Let's look at what substances belong to proteins.
The leader among proteins that are quickly digested is the egg. It should be eaten after training, because the egg does not leave behind a layer of fat like, for example, other animal proteins.
Fish is an excellent source of protein, it is easily digestible and does not cause heaviness in the stomach. As for animal meat, it is the undoubted leader in the amount of protein per 100 g. And if you eat 250 g of meat, then our body will receive the daily requirement of protein. But poultry meat compromises somewhat on its protein content, but it is lean and easier to digest.
As for plant proteins, the leader is soybean, which contains almost the same protein content as an egg. You should add nuts and seeds to your diet, the protein percentage of which reaches the same level as poultry meat, but due to their high calorie content they need to be consumed little by little.
The classification of proteins based on their physicochemical and chemical characteristics. Proteins are classified according to several criteria.
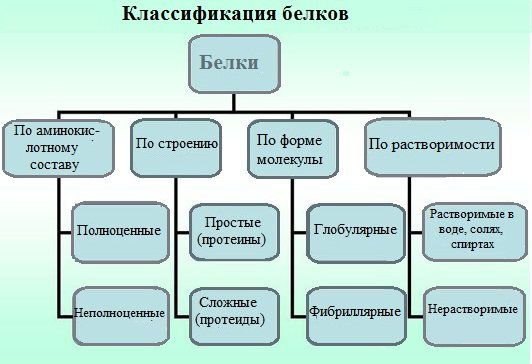
1.By structure
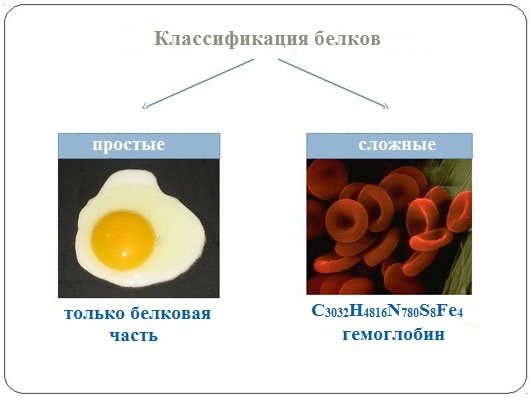
Based on the chemical structure of their molecules, all proteins are divided into simple and complex.
Simple proteins (proteins) consist only of amino acids.
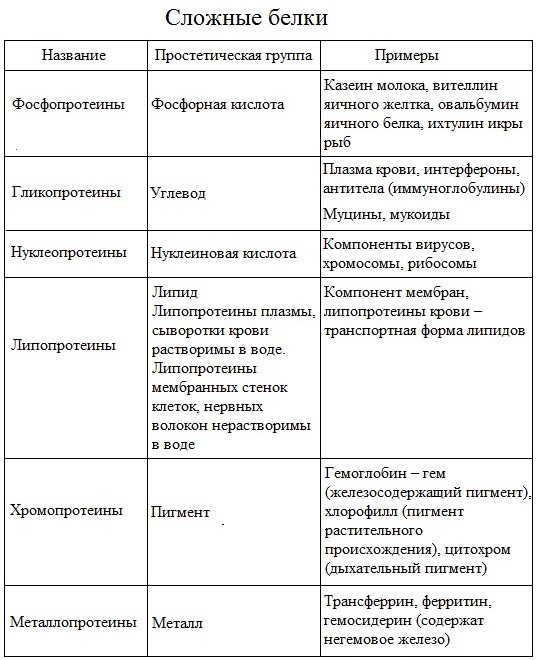
Complex proteins (proteids) consist of globular proteins and a non-protein component. The non-protein part of a complex protein is called a prosthetic group.
The prosthetic group can be represented by compounds of different chemical nature. Depending on its structure and properties, complex proteins are divided:
- chromoproteins – contain a colored component as a non-protein part (hemoglobin, myoglobin, cytochromes, chlorophyll);
- glycoproteins – contain carbohydrates;
- nucleoproteins – contain nucleic acids;
- lipoproteins – contain lipids;
- phosphoproteins - contain an orthophosphoric acid residue;
- metalloproteins – contain complexly bound metal.
Simple proteins
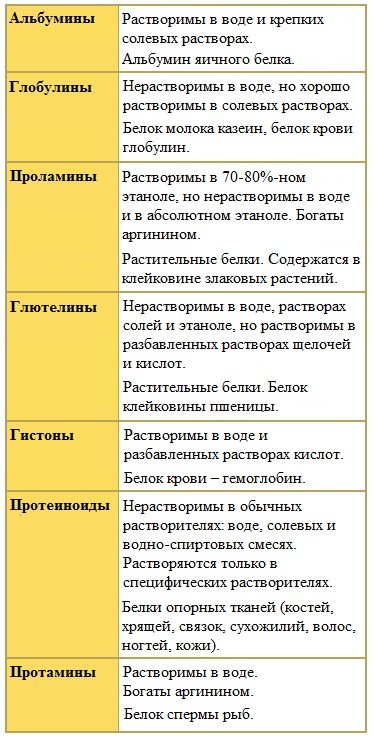
Simple proteins include albumins, globulins, protamines, histones, prolamins, glutelins, and proteinoids .
Albumins and globulins are proteins that are found in all tissues. Blood serum is the richest in these proteins. Albumin accounts for more than half of the proteins in blood plasma.
Albumin
Albumins constitute the main part of proteins in animal and plant tissues. Albumins are globular proteins.
Albumins are proteins with a relatively small molecular weight of 25,000-70,000; they have a pronounced acidic character, as they contain large amounts of aspartic and glutamic acids.
They dissolve in pure water and dilute solutions of acids, alkalis and salts. From aqueous solutions, albumins are precipitated by ammonium sulfate only when the solution is completely saturated, because these are highly hydrated proteins.
When boiled, they coagulate and precipitate in the form of thick flakes of denatured protein. The formation of foam on milk and thickening of the contents of eggs during cooking is explained by the denaturation of albumins. The foam formed when cooking fruits and vegetables consists partly of coagulated plant albumins.
Albumins are proteins primarily of animal origin. These include blood serum albumin, milk lactalbumin, egg white ovalbumin, animal muscle myoalbumin, as well as leukosin from wheat, rye and barley, legumenin from buckwheat and soybeans, and ricin from castor beans.
Albumins perform nutritional, transport, and neutralizing functions in the body.
A characteristic property of albumins is their high adsorption capacity. They adsorb polar and non-polar molecules, performing a transport role.
They transport hormones, cholesterol, bilirubin, drugs, and calcium ions.
Albumin binds toxic compounds - alkaloids, heavy metals, bilirubin.
Due to their high hydrophilicity, small molecular sizes, and significant concentration, albumins play an important role in maintaining the osmotic pressure of the blood. Albumin provides 80% of the blood osmotic pressure of all other serum proteins.
Albumin is synthesized mainly in the liver and is rapidly renewed.
Globulins
Globulins are a widespread group of globular proteins, usually accompanying albumins. Globulins have a higher molecular weight than albumins. Globulins are weakly acidic or neutral proteins.
Globulins are soluble in weak saline solutions, insoluble in distilled water and precipitate when solutions are 50% or more saturated with ammonium sulfate; they coagulate when heated.
Globulins include whey, milk, egg, muscle and other globulins.
There are many globulins in food products. Peas contain the protein legumin, soybeans - glycipin, bean seeds - phaseolin, potatoes - tuberin, blood - fibrinogen, milk - lactoglobulin, eggs - egg globulin, hemp - edestin.
Globulins in the body perform nutritional, protective, and transport functions.
In the blood, globulins transport cholesterol, phospholipids, triglycerides, iron (Fe2+), copper (Cu2+) ions, and vitamin B12. In milk, lactoglobulins and lactalbumins also perform a transport function.
Globulins are produced by the liver and immune system.
Protamines
Protamines are low molecular weight positively charged nuclear proteins with pronounced basic properties (alkaline proteins), with a low molecular weight of 4000–12000, containing 60-85% arginine.
Protamines are an integral part of many important complex proteins (nucleoproteins) that make up the cell nucleus. In the nuclei of cells they are in complex with DNA.
Protamines dissolve well in water, acidic and neutral media and precipitate in alkaline media; they do not precipitate when boiling.
Protamines are found in the nuclei of spermatozoa in fish. They constitute the major protein fraction in mature fish sperm.
Protamines are contained in the sperm of some fish species (salmin - salmon, klupein - herring), mackerel - mackerel.
They perform mainly a structural function, which is why they are present in cells that are not capable of dividing.
Histones
Histones are low molecular weight (11000–22000) proteins with a tertiary structure and have pronounced basic (alkaline) properties, because contain large amounts of arginine and lysine.
Histones are contained in the nuclei of cells of higher organisms in combination with nucleic acids, forming nucleoproteins.
Histones play an important role in the regulation of gene activity. These are chromosome proteins; they are part of the chromatin structure. In cells, positively charged histones are associated with negatively charged DNA in chromatin. Histones in chromatin form a scaffold onto which the DNA molecule is wound.
These are very stable proteins, the molecules of which can persist throughout the life of the cell.
Histones are found in the form of nucleoproteins in leukocytes and red blood cells (hemoglobin).
Histones are similar in properties to protamines; they are soluble in water and dilute acids, insoluble in aqueous ammonia and do not coagulate when heated. Histone molecules are polar and very hydrophilic, so they are difficult to salt out of solutions.
The main functions of histones are structural and regulatory.
Structural - histones are involved in stabilizing the spatial structure of DNA, and therefore chromatin, chromosomes and nucleosomes.
Regulatory – is the ability to block the transfer of genetic information from DNA to RNA.
Prolamins
Prolamins are proteins of plant origin, found in the gluten of cereal seeds, where they act as storage proteins. They contain large amounts of glutamic acid and proline (hence the name prolamine).
Prolamins contain almost no glycine and lysine, which makes their nutritional value low.
A characteristic feature of prolamins is that they are insoluble in water, saline solutions, alkalis, and are highly soluble in a 60-80% solution of ethyl alcohol (this is due to the presence of a large amount of the non-polar amino acid proline), while all other proteins denature and precipitate into sediment.
These include gliadin (wheat and rye protein), hordein (barley protein), zein (corn protein), avenin (oat protein), edestin (hemp protein).
Prolamins are practically absent in legumes and oilseeds.
Glutelins
Glutelins are proteins of plant origin, characterized by a high content of the amino acids proline and glutamic acid.
Glutelins play an important role in human nutrition because their nutritional value is high. They are present in cereal seeds along with prolamines.
Glutelins occupy an intermediate position between prolamins and globulins.
Glutelins are soluble in dilute acids and alkalis, but insoluble in water, alcohol and dilute saline solutions.
Representatives of this class of simple proteins are oryzenin (rice protein), glutelin (corn protein) and glutenin (wheat protein).
In rice, 80% of the total protein comes from glutelins (orysenin), this can explain the high lysine content in the protein of the rice grain.
These proteins in rye flour do not form gluten, which is due to the qualitative difference between the proteins of rye and wheat.
Proteinoids
Proteinoids are fibrillar proteins; their molecules form multimolecular thread-like complexes - fibrils.
Proteinoids are proteins of animal origin, rich in glycine, proline, and cystine. They can have tertiary and quaternary structures.
Proteinoids are proteins of supporting tissues (bones, cartilage, tendons, ligaments). They are represented by collagen, elastin and keratin.
Proteinoids do not dissolve in water, saline solutions, diluted acids and alkalis. They are not digested in the gastrointestinal tract of most animals and humans and therefore cannot perform a nutritional function. However, some arthropods have adapted to feeding on fibrillar proteins of skin, bird feathers, and wool (for example, moths).
Proteinoids include collagen - the main protein of skin, bones and cartilage, elastin - the protein of tendons and connective tissue, keratin - the protein of hair, wool, hooves, horns and silk fibroin .
Collagen
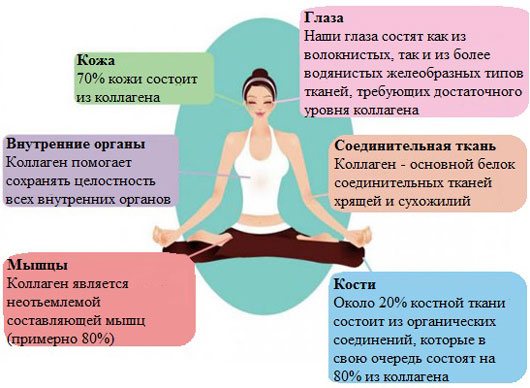
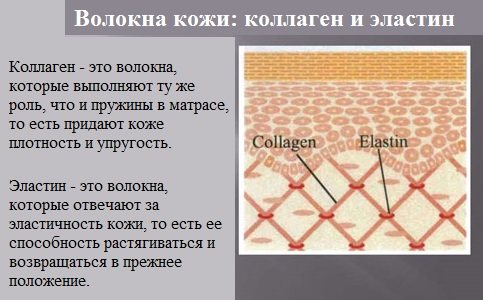
Collagen is the main protein of connective tissue of animals and humans, consisting of three protein strands twisted into a spiral. Collagen protects tissues from mechanical stress, maintaining the strength of the skin.
Collagen is a widely distributed protein in the body, making up about a third of all proteins in the body. More than 80% of the body’s total collagen is found in the intercellular substance of the connective tissue of the skin, bones, ligaments, tendons, and cartilage. These fabrics have low elongation and high strength.
The peculiarities of the amino acid composition of collagen include, first of all, a high content of glycine and proline. The polypeptide chains of collagen contain about 1000 amino acids.
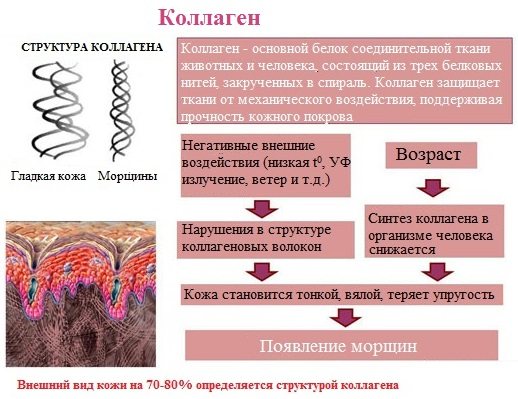
Collagen, heated for a long time in water at 56-1000C, turns into soluble glue, or glutin (gelatin), which, when cooled, hardens and forms jelly. The preparation of jellied dishes is based on this property of gelatin.
Elastin
Elastin is the main protein of elastic fibers, which are found in large quantities in the intercellular substance of tissues such as skin, blood vessel walls, ligaments, and lungs. These fabrics have very important properties: they can stretch several times their original length, while maintaining high tensile strength, and return to their original state after the load is removed.
Elasticity is associated with the presence in elastin of a large number of interchain cross-links with the participation of the amino acid lysine.
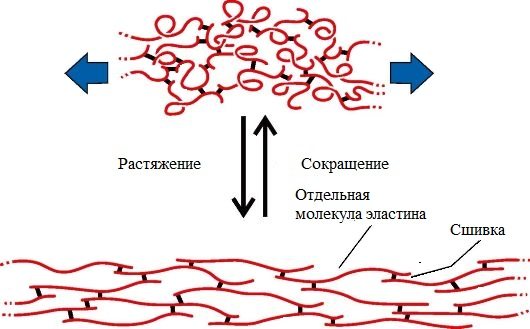
Elastin is insoluble in water and cannot swell. Elastin contains many hydrophobic amino acids - glycine, valine, alanine, leucine, proline.
Keratin
Keratins are a family of fibrillar proteins with mechanical strength that is second only to chitin among materials of biological origin.
Animal hair (fur), nails, feathers, quills, claws, horns and hooves are composed primarily of keratin.
Keratins can have an α-structure and a β-structure.
α-Keratin is a structural protein, built primarily in the form of an α-helix.
In α-keratins, three α-helices combine to form a superhelix. α-keratin molecules are oriented in parallel and connected by disulfide bonds (contain a lot of cysteine), which gives strength to the structure.
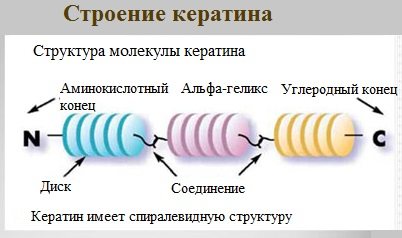
An example of β-keratins is silk fibroin.
Keratins are insoluble in solutions of salts, acids, and alkalis. Their molecular weight is very high.
Silk fibroin
Silk fibroin is a fibrillar protein secreted by arachnids and some insects and forms the basis of spider web threads and insect cocoons, in particular, silkworm silk.
Its β-structure consists of antiparallel polypeptide chains linked by hydrogen bonds. Fibroin consists mainly of glycine, alanine, serine, and tyrosine.
Complex proteins
Phosphoproteins
Phosphoproteins are complex proteins whose prosthetic group is a phosphoric acid residue . It binds to the peptide chain through tyrosine, serine and threonine residues, i.e. those amino acids that contain an OH group.
Proteins of this class include:
- milk casein, in which the phosphoric acid content reaches 1%;
- vitellin, vitellinin and phosvitin, isolated from chicken egg yolk;
- ovalbumin, discovered in chicken egg white;
- ichtulin, found in fish eggs and which plays an important role in the development of the fish embryo.
The biological role of phosphoproteins is that they are nutrients necessary for growing organisms.
Phosphoproteins are a valuable source of energy and plastic material for the development of the embryo and further growth and development of the body.
For example, casein (caseinogen) in milk contains all the essential amino acids and phosphoric acid. It also contains calcium ions.
Phosphorus and calcium are needed by a growing body in large quantities for the formation of the skeleton.
Glycoproteins
Glycoproteins (glycoconjugates) are complex proteins that contain a carbohydrate component as a prosthetic group.
In some glycoproteins, the carbohydrate part is loosely bound to the protein and can be easily separated from it. Prosthetic groups of some glycoproteins can be found in tissues and in a free state.
Glycoproteins are widely distributed in nature. They are found in secretions (saliva, etc.), as part of cell membranes, cell walls, intercellular substance, and connective tissue. Many enzymes and transport proteins are glycoproteins.
Glycoproteins are divided into true glycoproteins and proteoglycans.
True glycoproteins
The carbohydrate part of glycoproteins is represented by small heteropolysaccharides or oligosaccharides of irregular structure and contains mannose, galactose, glucose, and their amino derivatives. The protein in them makes up 80-85% of the mass of the macromolecule.
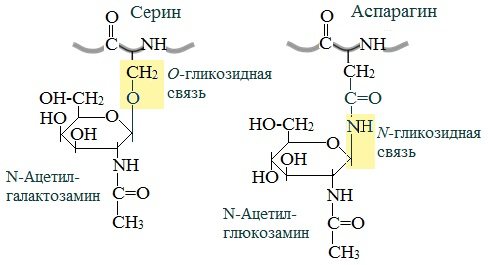
Glycoproteins are characterized by a covalent glycosidic bond. The N-glycosidic bond occurs between the carbohydrate component and the amide group of asparagine in proteins. For example, in immunoglobulins, enzymes and hormones).
O-glycosidic bond - the monosaccharide is connected to the OH group of serine or threonine (in mucins), and sometimes to the OH group of hydroxylysine or hydroxyproline (collagens).
Typical glycoproteins include most protein hormones, substances secreted into body fluids, complex membrane proteins, all antibodies (immunoglobulins), blood plasma proteins, milk, interferons, and blood groups.
Functions of glycoproteins
- Structural – collagen, elastin.
- Protective – antibodies (immunoglobulins), interferon, blood clotting factors (prothrombin, fibrinogen).
- Receptor – the attachment of an effector leads to a change in the conformation of the receptor protein, which causes an intracellular response.
- Hormonal – gonadotropic, adrenocorticotropic and thyroid-stimulating hormones.
- Enzymatic – enzymes: cholinesterase, nuclease.
- Transport – transfer of substances in the blood and through membranes (transferrin, transcortin, albumin, Na+,K+-ATPase).
Proteoglycans
A special group of glycoproteins consists of proteoglycans, in which the carbohydrate component predominates and accounts for 90% or more. Moreover, these substances are more similar in their properties to polysaccharides than to proteins.
The prosthetic group of proteoglycans is represented by heteropolysaccharides with a regular structure.
The carbohydrate part, similar to glycoproteins, binds to the protein through serine and asparagine residues.
Carbohydrate fragments enhance the hydrophilic properties of the protein due to the large number of OH groups and acidic groups. The chains of the latter are not flexible enough and tend to take on the conformation of a very loose random coil, occupying a huge volume.
Being hydrophilic, they attract a lot of water and even in low concentrations form hydrated gels. A similar ability creates turgor in the extracellular space.
Proteoglycans form the main substance of the intercellular matrix (intercellular space).
Cartilage matrix proteoglycans contain hyaluronic acid, which forms a gelatinous gel that acts as a shock absorber in cartilage and articular surfaces.
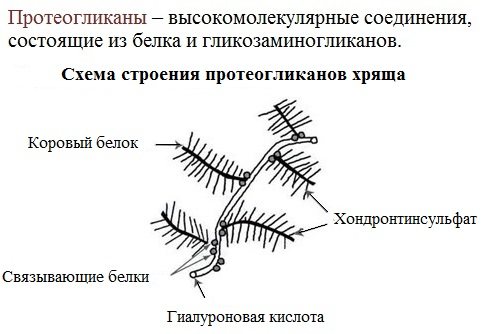
In terms of function, proteoglycans are important for the intercellular space, especially connective tissue, into which collagen fibers are immersed. They have a tree-like structure, with hyaluronic acid in the center.
Because their molecules are hydrophilic, they create a mesh jelly-like matrix and fill the space between cells, being a barrier to large molecules and microorganisms.
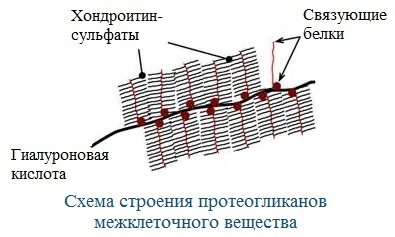
Various proteoglycans are present in the intercellular matrix. Among them there are very large ones - for example, aggrecan and vorsican.
In the intercellular space there is also a whole set of so-called small proteoglycans, which are widely distributed in different types of connective tissue and perform various functions there.
Based on the ratio of protein and carbohydrate parts, glycoproteins are divided into neutral and acidic.
Neutral glycoproteins include egg white (ovalbumin), blood plasma glycoproteins, and thyroid protein (thyroglobulin).
Acid glycoproteins include mucins and mucoids.
Mucins are the basis of body mucus (saliva, gastric and intestinal juice). They perform a protective function - they protect the walls of the digestive tract from mechanical and chemical damage. Mucins are resistant to the action of enzymes that hydrolyze protein.
Mucoids are proteins of the synovial fluid of joints, cartilage, and fluid of the eyeball. They perform a protective function and act as a lubricant in the movement apparatus.
The composition of acidic glycoproteins includes uronic acid, which takes part in the neutralization of bilirubin and drugs.
Nucleoproteins
Nucleoproteins (DNP and RNP) are complex proteins whose prosthetic group is nucleic acids (RNA and DNA).
Two types of nucleoproteins have been found in nature: deoxyribonucleoproteins (DNPs), complexes of proteins with deoxyribonucleic acid (DNA), and ribonucleoproteins (RNP), complexes of proteins with ribonucleic acid (RNA).
DNPs are predominantly localized in the nucleus and mitochondria, and RNPs are located in the cytoplasm, and high-molecular-weight RNPs are also found in the nucleus (nucleolus).
There are two types of nucleic acids depending on the pentose they contain - ribonucleic acid (RNA), if it contains ribose, and deoxyribonucleic acid (DNA), if it contains deoxyribose.
Differences between RNA and DNA
- number of chains: RNA has one chain, DNA has two chains;
- Dimensions: DNA is much larger;
- localization in the cell: DNA is in the nucleus, almost all RNA is outside the nucleus;
- type of monosaccharide: in DNA - deoxyribose, in RNA - ribose;
- nitrogenous bases: DNA contains thymine, RNA has uracil;
- function: DNA is responsible for storing hereditary information, RNA is responsible for its implementation.
DNA is predominantly concentrated in the nucleus of cells in chromosomes, mitochondria and chloroplasts.
Functions of DNA
Storage, reproduction and inheritance of genetic material, gene expression.
There are three main types of RNA:
- matrix (information) – mRNA (mRNA) is contained in the nucleus and cytoplasm.
- transport - tRNA is mainly found in the cytoplasm of the cell.
- ribosomal - rRNA makes up an essential part of the ribosome.
Functions of RNA
mRNA (mRNA) - reads information from a section of DNA about the primary structure of the protein and carries this information to ribosomes (carries information from the nucleus to the cytoplasm).
tRNA – transports amino acids to the site of protein synthesis (from the cytoplasm to the ribosomes).
rRNA is part of ribosomes (the ribosome framework is built from it) and participates in the synthesis of protein (polypeptide) chains.
RNA in some viruses carries genetic information instead of DNA.
Video “Nucleic acids in protein biosynthesis”
Lipoproteins
Lipoproteins are complex proteins whose prosthetic group is represented by some kind of lipid.
Lipids play an important role in the human body. They are found in all cells and tissues and participate in many metabolic processes.
They form the structural basis of all biological membranes and are present in a free state mainly in blood plasma and lymph.
Plasma and serum lipoproteins are soluble in water. Lipoproteins of the membrane walls of cells and nerve fibers are insoluble in water.
Lipoproteins can simultaneously contain free triglycerides, fatty acids, neutral fats, phospholipids and cholesterol (cholesterol).
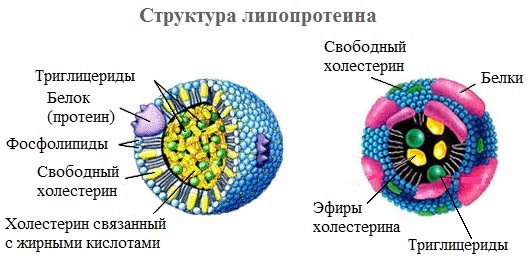
All types of lipoproteins have a similar structure: a hydrophobic core and a hydrophilic layer on the surface. The hydrophilic layer is formed by proteins (apoproteins), phospholipids and cholesterol. Triacylglycerols (TAGs) and cholesterol esters make up the hydrophobic core .
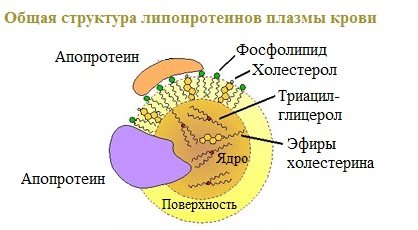
The hydrophilic groups of these molecules are oriented towards the aqueous phase, and the hydrophobic parts are oriented towards the hydrophobic core of the lipoprotein, in which the transported lipids are located.
Lipids do not dissolve in water, therefore they cannot be transported by blood in their pure form. Therefore, for the transport of lipids in the blood, complexes of lipids with proteins are formed in the body - lipoproteins.
The following types of lipoproteins are synthesized in the body: chylomicrons (CM), very low density lipoproteins (VLDL), intermediate density lipoproteins (IDL), low density lipoproteins (LDL) and high density lipoproteins (HDL).
Each type of lipid is formed in different tissues and transports specific lipids.
The common function of all lipoproteins is lipid transport.
Lipoproteins are highly soluble in blood because they are small in size and have a negative charge on the surface. Some lipoproteins easily pass through the walls of capillaries of blood vessels and deliver lipids to cells.
The large size of chylomicrons does not allow them to penetrate the walls of the capillaries, so from the intestinal cells they first enter the lymphatic system and then flow through the main thoracic duct into the blood along with the lymph.
Very low and low density lipoproteins cause atherosclerosis when their concentration in the blood increases.
When lipid transport and lipid metabolism are impaired, the body's energy potential decreases, the transmission of nerve impulses deteriorates, and the rate of enzymatic reactions decreases. Without the participation of lipoproteins, the transport of fat-soluble vitamins is impossible: vitamins of groups A, E, K, D.
Chromoproteins
Chromoproteins (“colored proteins”) are complex proteins containing a colored component as a prosthetic group.
Chromoproteins are involved in vital processes such as photosynthesis, respiration, transport of oxygen and carbon dioxide, redox reactions, light and color perception, etc.
Depending on their structure, hemoproteins, flavoproteins, and rhodopsin are distinguished.
Hemoproteins (red) are complex proteins whose prosthetic group is heme.
The group of hemoproteins includes hemoglobin, myoglobin, chlorophyll-containing proteins and enzymes (cytochromes, catalase and peroxidase). All of them contain iron (or magnesium) porphyrins as a non-protein component, but proteins are different in composition and structure, and perform various biological functions.
Chlorophyll (magnesium porphyrin), together with protein, ensures the photosynthetic activity of plants by catalyzing the splitting of water molecules into hydrogen and oxygen (by absorbing solar energy). Hemoproteins (iron porphyrins), on the contrary, catalyze the reverse reaction - the formation of a water molecule associated with the release of energy.
Hemoglobin , the main component of red blood cells and the main respiratory pigment, ensures the transfer of oxygen ( O2 ) from the lungs to the tissues and carbon dioxide ( CO2 ) from the tissues to the lungs. Maintains acid-base balance of blood.
In hemoglobin, the protein component is represented by globin, and the non-protein component is heme - pigment. The iron ion is located at the center of the heme pigment, which gives blood its characteristic red color. Heme is represented by porphyrin, consisting of 4 pyrrole rings. Each of the 4 heme molecules is “wrapped” by one polypeptide chain.
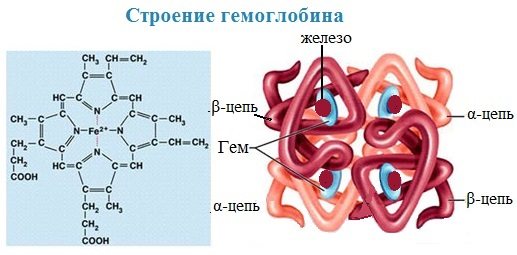
Heme is also a prosthetic group in myoglobin, catalase, peroxidase, and cytochromes. Heme is also found in plant hemoproteins and is involved in the process of photosynthesis.
Myoglobin (muscle protein) is a small globular protein; its molecule consists of one polypeptide chain and one heme. Myoglobin creates an oxygen reserve in the muscles that is used by muscle fibers.
Chromoproteins also include flavoproteins, the prosthetic groups of which are isoalloxazine derivatives. Flavoproteins are part of oxidoreductases - enzymes that catalyze redox reactions in the cell. Some flavonoids include metal ions and a heme molecule.
Rhodopsin is a protein whose prosthetic group is the active form of vitamin A, retinal. Rhodopsin is the main photosensitive substance of the retinal rods. Its function is to perceive light at dusk, i.e. responsible for twilight vision.
Metalloproteins
Metalloproteins are complex proteins in which metal ions play the role of a non-protein component.
Metalloproteins include about a hundred enzymes.
An important function of metalloproteins is related to the transport of metals and their storage in the body.
Typical metalloproteins are proteins containing non-heme iron - transferrin, ferritin, hemosiderin, which are important in iron metabolism in the body.
Transferrin is a water-soluble iron protein found in blood serum as part of β-globulins. The transferrin molecule contains two Fe3+ ions. This protein serves as a carrier of iron in the body. Transferrin is synthesized in the liver.
Ferritin is an intracellular globular protein, found mainly in the spleen, liver, and bone marrow, serving as an iron depot in the body. Thanks to ferritin, cytosolic iron stores are maintained in a soluble and non-toxic form.
Hemosiderin, unlike ferritin and transferrin, is a water-insoluble iron-containing protein complex. It is found mainly in the cells of the liver and spleen, and accumulates when there is excess iron in the body, for example, with frequent blood transfusions.
Ceruloplasmin is a whey protein that contains copper and takes part in its metabolism, as well as the metabolic processes of iron. Refers to α-2-globulins.

Catalase - neutralizes hydrogen peroxide.
Cytochrome oxidase - in combination with other enzymes of the mitochondrial respiratory chain, participates in the synthesis of ATP.
Alcohol dehydrogenase - ensures the metabolism of ethanol and other alcohols
Lactate dehydrogenase - participates in the metabolism of lactic acid
Carbonic anhydrase - forms carbonic acid from CO2 and H2O.
Xanthine oxidase is responsible for the final reactions of catabolism of purine bases.
Thyroid peroxidase is involved in the synthesis of thyroid hormones.
Glutathione peroxidase is an antioxidant enzyme.
Urease - responsible for the breakdown of urea.
2. According to the shape of the molecules (fibrillar and globular)
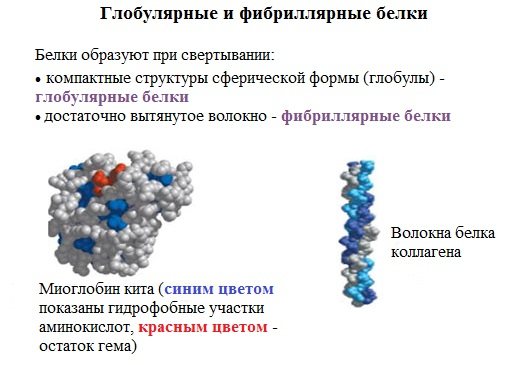
Proteins can be classified according to the shape of their molecules and some physical properties into two large classes: fibrillar and globular proteins.
Fibrillar proteins are long filament-like molecules, the polypeptide chains of which are located parallel to each other along one axis and form long fibers (fibrils) or layers.
The most important is the secondary structure (the tertiary structure is almost not expressed at all).
Most fibrillar proteins are insoluble in water and have a large molecular weight.
These proteins are characterized by high mechanical strength and perform a structural function.
Fibrillar proteins include keratins (hair, wool, horns, hooves, nails, feathers), myosin (muscles), collagen (tendons and cartilage), fibroin (silk, spider webs).
Globular proteins are characterized by a compact three-dimensional arrangement of polypeptide chains; their molecules have a globule shape.
The tertiary structure is most important.
Globular proteins dissolve in water or dilute saline solutions. Due to the large size of the molecules, these solutions are colloidal.
Globular proteins perform the functions of enzymes, antibodies (blood serum globulins determine immunological activity) and, in some cases, hormones (insulin).
They play an important role in protoplasm, holding water and some other substances in it and helping to maintain molecular organization.
Globular proteins are found in physiological fluids (blood serum, milk, digestive fluids) and in body tissues.
There are also intermediate proteins of fibrillar nature, but soluble. An example is fibrinogen, which is converted into insoluble fibrin during blood clotting.
3.By solubility in individual solvents
The classification of simple proteins is based primarily on solubility in water, alcohol, saline solutions, solutions of alkalis and acids.
4.According to amino acid composition
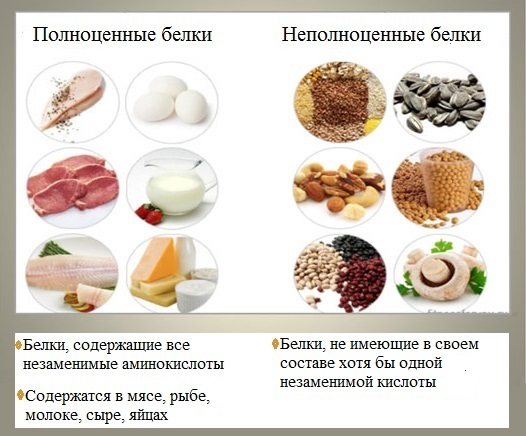
From the point of view of the nutritional value of proteins, determined by their amino acid composition and the content of essential amino acids, proteins are divided into complete and incomplete .
Proteins are considered complete if they contain eight essential amino acids that the body cannot synthesize on its own.
Incomplete proteins are those that contain insufficient amounts of one or more essential amino acids that cannot be synthesized by the body.
Complete proteins are found in animal products (except gelatin), as well as some plant foods (peas, beans, soy).
Incomplete proteins are mainly of plant origin.
Squirrels
Use of proteins, carbohydrates and fats in the diet
If we approach nutrition from a scientific point of view, then a person does not require specific food products, but their elements for the growth and functioning of the body. These are 3 groups of substances: proteins, fats, carbohydrates. All of them must be present in a specific amount every day, otherwise disruptions in the body and deterioration in health are possible. There is also a certain ratio between these substances that must be adhered to.
Nutritionists recommend building your diet taking into account the following norm: 15% proteins, 20% fats and 65% carbohydrates. Of course, this is an average proportion: it depends on the specific organism, height parameters, weight and age of the person. Therefore, subjective numbers should be determined by contacting a nutritionist or trainer.
Features of protein nutrition for muscle growth
For any intense sports, it is important to increase muscle mass and increase the body's endurance. This is achieved through intensive training and a special diet that includes foods high in protein.
It would be best if the protein nutrition menu is drawn up by the athlete’s doctor or trainer. It is important to correctly calculate the protein diet, the number of calories, carbohydrates and fats.
An athlete's protein diet should include: low-fat dairy products, lean meat, boiled egg whites, and low-fat sea fish. Meals should be fractional - 5 times a day. After an intense workout, it is recommended to take a protein shake.
During the period of muscle mass gain, the percentage of biological substances is as follows: 70% - protein, 30% - fats and carbohydrates. The maximum duration of a protein diet should be no more than 1 month. Longer than this time, its use can cause harm to the body.
The work of proteins
High-molecular biological compounds, which include proteins, are broken down into amino acids, which penetrate into the blood and saturate the body, and it uses them to build its proteins. In order for the body to acquire strong muscles and a beautiful, toned appearance, it is necessary to replenish the diet with proteins. There are enough of them in fish and meat, eggs and cheese, legumes and soy products. The diet should be calculated based on the daily intake of all ingredients, as well as the goals set.

Those who want to pump up their muscles should increase their protein intake, and those who are losing weight should reduce their intake of high-carbohydrate and fatty foods. Proteins, fats, carbohydrates are the main sources of energy and strength. On average, for a healthy diet and maintaining a normal weight, a person needs to take proteins, fats, and carbohydrates with food in the following proportion: 90-110/90-100/250-300 g. For children, these numbers are slightly lower.
Protein foods and daily servings:
- chicken, turkey, lean ham - 75 g;
- pork, lamb, beef - 45 g;
- fatty fish – 30 g; lean fish, seafood – 60 g;
- beans, lentils – 2 tbsp. l.;
- bacon - 1 lean slice;
- fish sticks – 2 pcs.;
- low fat hummus – 1 tbsp. l.;
- eggs - 1 pc.;
- nuts - 15 g.
Muscles are built from protein, and human food must contain it, because it is a source of strength. Proteins from fish and poultry are best absorbed. Products such as legumes, cereals, and soy also contain proteins, and, moreover, a small amount of fat; cheeses and dairy products are not far behind them.
Functions of proteins in the body
1. Plastic (structural) function. All human tissues and organs are built from proteins; they give shape to the cell and its components.
2. Hormonal and enzymatic function. Proteins are the basis of all enzymes (catalysts), without which the absorption of food and all biochemical reactions in the body are impossible.
Hormone proteins regulate the concentration of substances in the blood and cells, growth, reproduction and many other processes. For example, the protein insulin regulates blood sugar concentrations and sends sugar to the muscles or liver.
3. Protective function. The protein collagen creates the basis of the skin, which protects the body from the environment; enzyme proteins break down toxins in the liver; Blood proteins form the basis of the body's immune system and protect against viruses, bacteria and foreign proteins.
4. Transport function. Proteins ensure the transport of various mineral salts and vitamins through cell membranes and intracellular structures, and are involved in the transport of oxygen, lipids, carbohydrates, some vitamins, hormones and drugs.
5. Distribution function. Proteins are involved in the distribution of fluid between the intra- and extracellular environment in the body.
6. Energy function. When more protein is consumed than is needed by the body's tissues and cells, the human body uses it as an energy resource. This function is activated when there is a lack of other energy sources - carbohydrates and fats.
7. Motor function. The proteins actin and myosin provide muscle movement.
Interesting facts about proteins
More than 4 billion years ago on planet Earth, proteins incredibly emerged from insignificant inorganic molecules, which became the origin of life. All living things exist thanks to unique protein molecules, and other forms of life in the Universe are still unknown to scientists.
- Every living organism is made of proteins. They occupy about 50% of the dry matter of any organism. Viruses contain protein from 45 to 95%.
- About 30% of a person's protein comes from muscles, about 20% from bones and tendons, and about 10% from skin.
- There are 1012 different protein substances that give life to organisms of all levels of complexity, from viruses to humans.
- The brain is also a protein. It should be remembered that due to the process of protein denaturation, when ethyl alcohol enters the body, brain cells die.
- Squirrels owe their name to egg white, which has been used by people since ancient times as a component of food.
- The role of proteins in the body is very diverse; they can act as enzymes. Pepsin is an enzyme that breaks down proteins into amino acids in the body.
- Proteins have a protective effect. Interferon is a protein that saves the human body from the invasion of viruses.
- Proteins can play the role of hormones, an example is the hormone insulin. It promotes the entry of glucose into the cell.
- Proteins fulfill the energy task for the body.
- Hair is made of pure protein.
What is protein
Protein is a complex organic compound that consists of amino acids. There can be from hundreds to thousands of them in protein. It is the variety of these small bricks that determines what kind of protein it will be and how healthy our body will be.
There are only 20 amino acids. By analogy with the book, we have only 33 letters of the alphabet, and how eloquent our language is. Is it true? There are 8 essential amino acids, which can only be obtained from food, and 2 conditionally essential amino acids. If you present it visually, this is the entire palette of protein products that we know.
Protein structures
There are 4 spatial organizations of protein molecules:
- primary - created by peptide bonds, it is this that determines the properties of protein molecules;
- the secondary, shaped like an extended spring, is built from peptide and hydrogen bonds (tendons, nails, hair, cobwebs);
- tertiary, spherical in shape, built from peptide, hydrogen and disulfide bonds (enzymes, antibodies, hormones);
- quaternary - consists of alpha and beta chains (hemoglobin).
Each protein is assigned a genetic code, which stores information about what form it should take, but geneticists still cannot predict its spatial structure from the primary code. For a protein to work, it needs to fold in a certain way.
It is very important to know which foods contain protein, what kind it is and how much it should be consumed. Why? But because protein, burning in the body, saturates it with a large amount of energy. It is after high-protein food that we feel a surge of strength; it is protein that builds each of our muscles and creates a beautiful and healthy body. Equally important is the fact that protein helps you lose weight or maintain weight after losing weight.
List of foods containing protein
| Food product | Proteins (g) per 100 g of product |
| Seafood | |
| Red caviar | 31,6 |
| Pink salmon | 23 |
| Salmon | 20 |
| Halibut | 20 |
| Shrimps | 18,7 |
| Perch | 18,5 |
| Saira in oil | 18,4 |
| Herring | 18 |
| Squid | 18 |
| Navaga, cod | 17,8 |
| Pollock | 17,7 |
| Flounder | 17,5 |
| Sprats (canned) | 17,3 |
| Sturgeon | 15,8 |
| Meat products | |
| Boiled chicken | 25 |
| Chicken breast | 23 |
| Chicken liver | 22 |
| Chicken | 20,5 |
| Beef | 21 |
| Beef | 21 |
| Pork | 20,5 |
| Pork liver | 20,2 |
| Mutton | 20 |
| Beef liver | 19,8 |
| Beef stew | 16,7 |
| Pork stew | 15 |
| Eggs | |
| Chicken egg | 12,9 |
| Quail egg | 11,9 |
| Dairy | |
| Parmesan cheese | 35 |
| Dutch cheese | 26 |
| Whole milk powder | 25 |
| Russian cheese | 23 |
| Low-fat cottage cheese | 18 |
| Fat cottage cheese | 14 |
| Processed cheese | 12 |
| Condensed milk with sugar | 7,2 |
| Yogurt | 3-4,5 |
| Kefir | 3,3 |
| Whole milk | 3,3 |
| Pasteurized milk | 3 |
| Sour cream 10% fat | 3 |
| Cream 10% fat | 3 |
| Cream 20% fat | 2,8 |
| Sour cream 20% fat | 2,8 |
| Nuts and legumes | |
| Soy flour | 37 |
| Soybeans | 36 |
| Peanut | 26,2 |
| Pumpkin seeds | 24 |
| Lentils | 23,5 |
| Sunflower seeds | 22,5 |
| Beans | 21 |
| Peas | 21 |
| Pistachios | 20 |
| Chickpeas | 20 |
| Almond | 18,8 |
| Cashew | 18,5 |
| Walnut | 16,2 |
| Hazelnut | 15 |
| Brazilian nut | 14,3 |
| Pine nut | 13,8 |
| Pecan | 9,2 |
| Cereals | |
| Wheat bran | 15 |
| Oat flour | 14 |
| Hercules | 13 |
| Couscous | 13 |
| Buckwheat | 12,5 |
| Wheat groats | 12,5 |
| Bulgur | 12 |
| Millet | 12 |
| Oat groats | 12 |
| Semolina | 11 |
| Buckwheat flour | 11 |
| Wheat flour 1st grade | 10,6 |
| Premium wheat flour | 10 |
| Barley grits | 10 |
| Pearl barley | 9 |
| Corn (whole kernels) | 8,5 |
| Corn flour | 8 |
| Rye grain bread | 8,5 |
| Wheat bread (white) | 7,6 |
| Brown rice | 7,5 |
| White rice | 7 |
| Boiled white rice | 2 |
| Vegetables | |
| Green peas | 5 |
| Brussels sprouts | 4,5 |
| Watercress | 4,2 |
| Broccoli | 3 |
| Spinach | 2,8 |
| Cauliflower | 2,5 |
Protein related products
It is not for nothing that protein diets are recognized as the most effective among nutritionists, because the body spends a lot of energy on processing protein foods, about 5-10% of incoming calories.
List of the highest protein foods.
- Chicken eggs contain 17% easily digestible protein. Nutritionists advise eating eggs after physical activity, but no more than 2 eggs per day to renew muscle tissue. At the same time, eggs are a low-calorie product.
- Low-fat cottage cheese contains 25% protein and is easily digestible.
- Hard cheese has about 30% protein, but remember that it is too high in calories, and the fatter it is, the less protein it contains.
- Poultry meat is the main source of dietary nutrition, since its calorie content is low and is easily digestible. Poultry meat contains from 15 to 20% protein.
- Beef is low in calories and generous in protein. Just one serving of lean beef contains about 22 grams of protein.
- The liver consists of 25% protein. Boiled and stewed, it is very useful, as it is rich in other essential elements.
- Some types of fish contain up to 25% protein, for example, tuna, salmon, mackerel. Such fish can be used for food without fear of gaining weight.
- The richest plant product in protein is soybeans, which contain 14% protein, while Brussels sprouts are the leading vegetable product (9%).
- Cereals contain at least 10% protein, which is perfectly absorbed by the body.

These are the main products related to proteins. The list given here is far from complete, but takes into account the body's basic protein needs.
Useful tips
- Do not overuse fatty fish and meats. The high fat content in a number of foods (beef brisket, pork, salmon, duck, cod liver, goose) complicates digestion and also interferes with the absorption of protein.
- Avoid shelf-stable meat products (sausages, ham, sausages, sausages) and semi-finished products. Frequent consumption of processed meats is perhaps the biggest problem that contributes to the development of protein starvation. It is well known that these products contain little pure meat, and in its current state it is almost not absorbed by the body.
- Optimal sources of protein are eggs, lean beef, and lean poultry. Plant proteins (nuts, buckwheat, peas, beans) should also be constantly present in the diet.
- Eat fish and meat separately from bread, potatoes and cereals. The best addition to protein foods is a vegetable salad (beets, carrots, cabbage).
- The healthiest way to cook meat for the body is barbecue or grilling. Thus, a large amount of fat, which overloads the gastrointestinal tract, is removed from the body.
- It is most beneficial to eat protein foods for dinner, since the body has time to fully absorb it overnight.
However, in everything you should adhere to the golden mean, which requires nutritional control.
Excessive consumption of foods containing a lot of protein leads to increased load on the liver and kidneys and overload of the digestive system.
Physicochemical characteristics
B. molecules have a mass from several thousand to 1 million and more. The sedimentation constant varies from 1 to 20 or more. The average specific volume of protein molecules is 0.70–0.75 cm3/g. B. molecules have a weak ability to diffusion and do not pass through semi-permeable membranes. The absorption maximum of B. in the UV region of the spectrum, due to the presence of aromatic compounds in their molecules. amino acid residues is located near 280 nm. In the IR region of the spectrum, B. absorbs due to the $\ce{COO}$- and $\ce{NH}$-groups at 1600 and 3100–3300 cm–1. Soluble bacteria are hydrophilic colloids that actively bind water. B. are amphoteric electrolytes, because they have free carboxyl and amine groups. Isoelectric points for different bacteria range from less than 1.0 (for pepsin) to 10.6 (for cytochrome c) and higher. B.'s solubility can also vary significantly. Some bacteria easily dissolve in water, others require small concentrations of salts for dissolution, and others go into solution only under the influence of strong alkalis or detergents. Different bacteria are differently precipitated from solutions by organic substances (for example, alcohols) or high concentrations of salts (salted out). Significant differences in solubility and other features are used to isolate individual proteins. Proteins give a number of color reactions due to the presence of certain amino acid residues or chemical groups. The most important of them include biuret (reaction with biuret, on a peptide bond) and ninhydrin (with ninhydrin, on an amino group) reactions. Side groups of amino acid residues are capable of entering into many chemical reactions. In this case, the reactivity of the same groups significantly depends on the position in the polypeptide chain of the protein: both on the localization of the group in the general spatial structure of the protein and on the influence of neighboring side groups. Groups located in the active center of the protein usually have the highest reactivity.
Protein production
Methods for isolating individual bacteria have been developed, based on Ch. arr. on chromatography, including affinity, size exclusion, ion exchange and hydrophobic. Their use in high-performance liquid chromatography mode is especially productive. Ultrafiltration, electrophoresis, etc. are also widely used. The criteria for biopurity are homogeneity during electrophoresis, chromatography, and ultracentrifugation. In B., consisting of one polypeptide chain, it is established by analyzing the N-terminal amino acid.
To obtain peptides, including hormones and their various analogues, as well as peptides carrying antigenic determinants of various types. B. and used for the preparation of appropriate vaccines, chemicals are widely used. synthesis. Chemical synthesis of some small biomass, but this very labor-intensive procedure still has more theoretical than practical significance. B., having industrial significance (enzymes, hormones, cytokines, interferons), are synthesized using recombinant DNA technology (genetic engineering) in foreign organisms and are products of biotechnological production Protein engineering methods make it possible to purposefully change the structure of proteins.