What are carbohydrates?
Carbohydrates are organic substances that are the main source of energy for your body. It is one of the three macronutrients you need to survive. The other two are proteins and fats .
Types of carbohydrates:
- Monosaccharides are the simplest carbohydrates that do not break down into even simpler ones. For example, glucose, fructose.
- Oligosaccharides are more complex compounds, built from several (up to 10) monosaccharide residues. For example, beet raffinose.
- Disaccharides are complex compounds built from 2 monosaccharide residues. For example, beet or cane sugar, lactose (milk sugar).
- Polysaccharides are complex compounds formed from a large number of glucose residues. They are divided into digestible (starch) and non-digestible (fiber). Fiber, due to its properties, has a beneficial effect on the entire body as a whole. Helps prevent many diseases, including cancer.
Carbohydrates
Carbohydrates are a group of natural organic compounds whose chemical structure corresponds to the formula Cm(H2O)n. They are part of all living organisms without exception.
Classification
Carbohydrates are divided into
- Monosaccharides
- Oligosaccharides
- Polysaccharides
Monosaccharides (Greek monos - single + sacchar - sugar) are the most common group of carbohydrates in nature, containing five (pentoses) or six (hexoses) carbon atoms in their molecules.
The most well-known representatives of pentoses include ribose and deoxyribose, and hexoses include glucose and fructose.
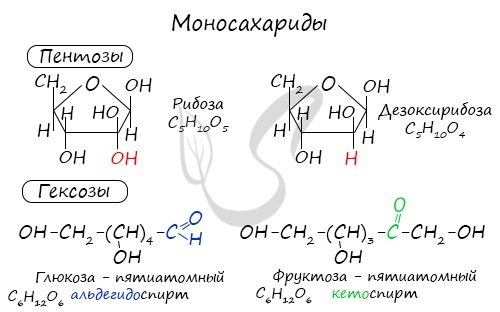
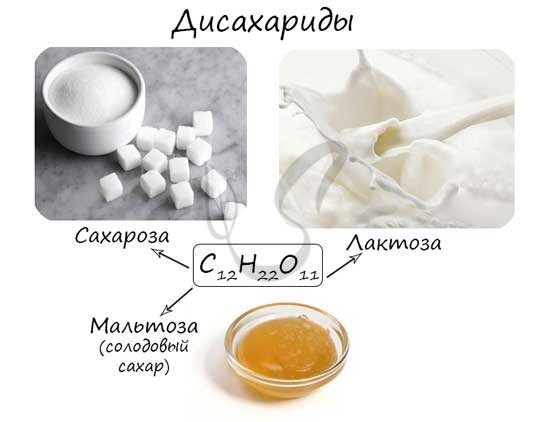
Oligosaccharides (Greek ὀλίγος - few) are a group of carbohydrates whose molecules contain from 2 to 10 monosaccharide residues. If a molecule contains two monosaccharide residues, it is called a disaccharide.
The most famous disaccharides are: sucrose, lactose, maltose. They are isomers, their molecular formula is the same - C12H22O11.
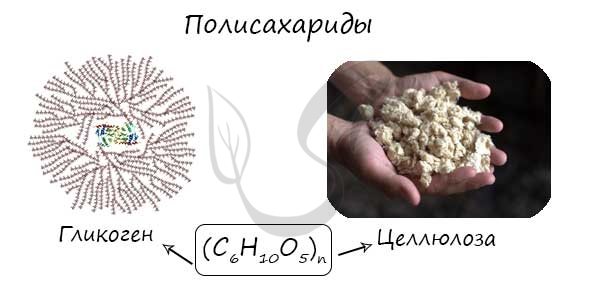
Polysaccharides (Greek poly - many) are natural biopolymers, the molecules of which consist of long chains (tens, hundreds of thousands) of monosaccharides.
For example, glucose is a monosaccharide, and starch, glycogen and cellulose are its polymers. Polymers also include chitin and pectin. Formula of starch, cellulose - (C6H10O5)n
Monosaccharides
Glucose can be obtained in several ways:
- Butlerov's reaction
- Starch hydrolysis
- Photosynthesis
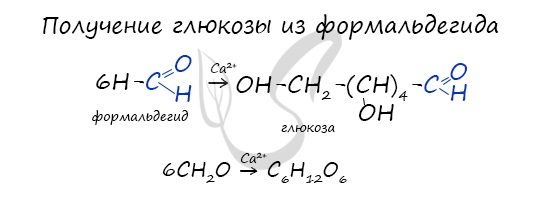
In the presence of metal ions, formaldehyde molecules combine to form various carbohydrates, such as glucose.
In the presence of acid and when heated, starch (polymer) breaks down into monomers - glucose molecules.
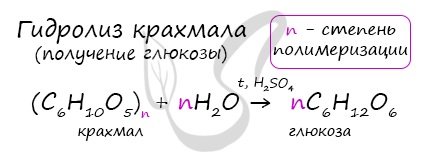
This reaction was invented by nature; there is an extraordinary catalyst for it - sunlight (hν).
6CO2 + 6H2O → (hν) C6H12O6 + 6O2↑
According to its chemical structure, glucose is a pentahydric aldehyde alcohol, which means that it is characterized by reactions of both aldehydes and polyhydric alcohols.
- Reactions by aldehyde group
- Reactions by hydroxo groups
- Glucose fermentation
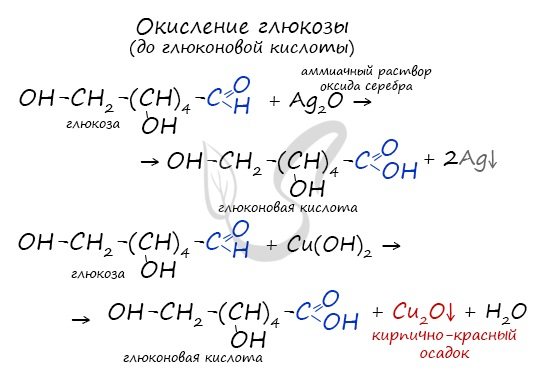
Glucose is oxidized to gluconic acid. This can be done using silver mirror reactions with copper II hydroxide.
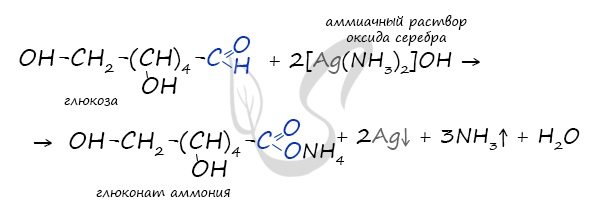
Pay special attention to the fact that when writing the formula of an ammonia solution in its entirety, it would be more correct to indicate in the products not the acid, but the salt - ammonium gluconate. This is because ammonia, which has basic properties, reacts with gluconic acid to form a salt.
Glucose can be reduced to the hexahydric alcohol sorbitol (glucite), which is used in the food industry as a sweetener. Sorbitol tastes less pleasant and less sweet than sugar.
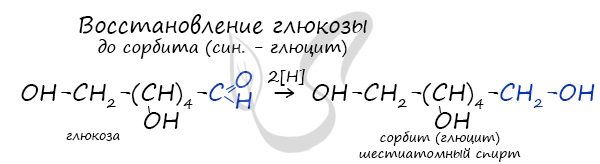
Glucose contains five hydroxyl groups and is a polyhydric alcohol. It enters into a qualitative reaction for polyhydric alcohols - with freshly prepared copper II hydroxide.
As a result of this reaction, a characteristic blue color of the solution is formed.

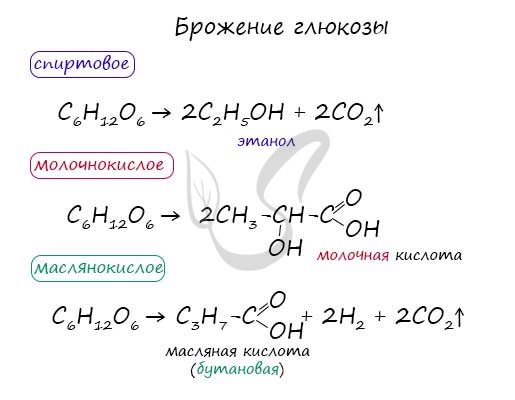
There are several options for fermentation of glucose: alcoholic, lactic acid, butyric acid. These types of fermentation are of great practical importance and are characteristic of many living organisms, in particular bacteria.
Fructose is an isomer of glucose. Unlike it, it does not enter into oxidation reactions - it is a keto alcohol, and ketones are not subject to oxidation to acids.
It is characterized by a qualitative reaction as a polyhydric alcohol - with freshly prepared copper II hydroxide. Fructose does not react with the silver mirror.
Fructose is used as a sweetener. It is 3 times sweeter than glucose and 1.5 times sweeter than sucrose.

Disaccharides
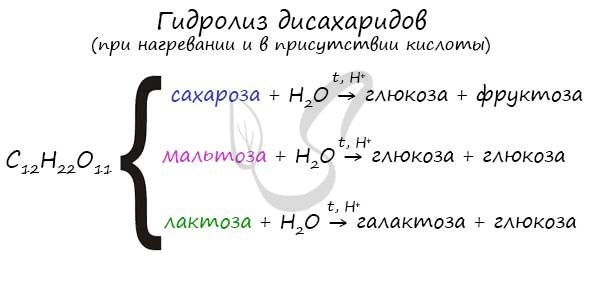
As mentioned earlier, the most famous disaccharides: sucrose, lactose and maltose have the same formula - C12H22O11.
Their hydrolysis produces various monosaccharides.
Polysaccharides
Of the many reactions, most of all I would like to highlight the hydrolysis of starch. As a result, glucose is formed.
© Bellevich Yuri Sergeevich 2018-2021
This article was written by Yuri Sergeevich Bellevich and is his intellectual property. Copying, distribution (including by copying to other sites and resources on the Internet) or any other use of information and objects without the prior consent of the copyright holder is punishable by law. To obtain article materials and permission to use them, please contact Yuri Bellevich
.
Functions of carbohydrates in the human body
- The role of carbohydrates is great. Once in the gastrointestinal tract, they are broken down into glucose, which in turn enters the cells and is used by the body as a source of energy. When they are insufficient to produce energy, proteins and fats break down, which leads to the accumulation of toxic ketones in the blood.
- They are able to accumulate in the liver, skeletal muscles, and other tissues in the form of glycogen.
- They take part in the synthesis of many substances necessary for the normal functioning of your body. For example, complex proteins, components of the immune system, etc.
- Regulates the metabolism of proteins and fats.
- Necessary for the normal functioning of the heart, liver, muscles and central nervous system.
Storage of glucose in the form of glycogen
The metabolism of carbohydrates in the body occurs as follows. After we eat something, the level of glucose in the blood rises and the pancreas is the first to react to this. It releases the hormone insulin, which signals the body's tissues to absorb excess glucose. Some of this glucose is used by muscle and liver cells to build the polysaccharide glycogen.
Muscles store 2/3 of the total amount of glycogen in the body and use it to provide their own nutrition during exercise. The remaining 1/3 is accumulated by the liver and is more generous in its distribution; When energy is depleted, it shares glycogen in the form of glucose in the blood with the brain and other organs.
When blood glucose concentrations drop and cells need energy, the bloodstream is flooded with pancreatic hormones, glucagons. Thousands of enzymes in liver cells release glucose into the blood to feed the rest of the body's cells. Another hormone, adrenaline, has a similar effect and is part of the body's defense mechanism during times of danger (the "fight or flight" response).
Although glucose can be converted to fat, body fat can never be converted back to glucose and provide normal nutrition to the brain. This is one reason why fasting or low-carb diets can be dangerous.
With a serious carbohydrate deficiency, the body faces two problems at once. First of all, due to a lack of glucose, it is forced to obtain it from proteins, thereby distracting them from such vital work as maintaining immune defense. The functions of proteins in the body are so irreplaceable that, just to avoid their use for energy, it is already worth maintaining the level of carbohydrates; this is called the "protein-sparing" effect of carbohydrates.
Also, without enough carbohydrates, the body cannot properly manage its fat reserves. (Fat fragments must combine with carbohydrates before they can be used to produce energy.) The minimum amount of carbohydrates required to fully protect protein and prevent ketosis for an average-sized person is about 100 g/day. And it’s better if these are easily digestible carbohydrates in an amount 3-4 times higher than this minimum.

What foods contain carbohydrates
Most foods are carbohydrates. They are not found in products of animal origin (meat, fish and seafood, eggs, etc.). The exception is dairy products that contain the milk sugar lactose.
Sources:
- Fruits.
- Vegetables, greens.
- Cereals, different types of flour.
- Nuts and seeds.
- Legumes (beans, peas, lentils, soybeans).
- Bread, pastries, cakes, pastries, etc.
- Pasta, noodles.
- Sugar, starch, honey.
- Carbonated drinks with sugar, compote, juices, tea and coffee with sugar.
- Alcohol.
- Dairy products, etc.
Chemical properties
Depending on their structure, each carbohydrate has special chemical properties. Monosaccharides, in particular glucose, undergo multi-stage oxidation (in the absence and presence of oxygen). As a result of complete oxidation, carbon dioxide and water are formed:
C6H12O6 + 6O2 → 6CO2 +6H2O.
In the absence of oxygen, fermentation occurs under the action of enzymes:
- alcoholic –
C6H12O6 → 2C2H5OH (ethanol) + 2CO2; - lactic acid –
C6H12O6 → 2CH3-CH(OH)-COOH (lactic acid).
Otherwise, polysaccharides interact with oxygen, burning to carbon dioxide and water:
(C6H10O5)n + 6O2 → 6nCO2 + 5nH2O.
Oligosaccharides and polysaccharides decompose to monosaccharides during hydrolysis:
- C12H22O11 + H2O → C6H12O6 + C6H12O6;
- (C6H10O5)n + nH2O → nC6H12O6.
Glucose reacts with copper(II) hydroxide and an ammonia solution of silver oxide (silver mirror reaction):
- CH2OH-(CHOH)4-CH=O + 2Cu(OH)2 → CH2OH-(CHOH)4-COOH + Cu2O↓ + 2H2O;
- CH2OH-(CHOH)4-CH=O + 2[Ag(NH3)2]OH → CH2OH-(CHOH)4-COONH4 + 2Ag↓ +3NH3 + H2O.

Rice. 3. Reaction of the silver mirror.
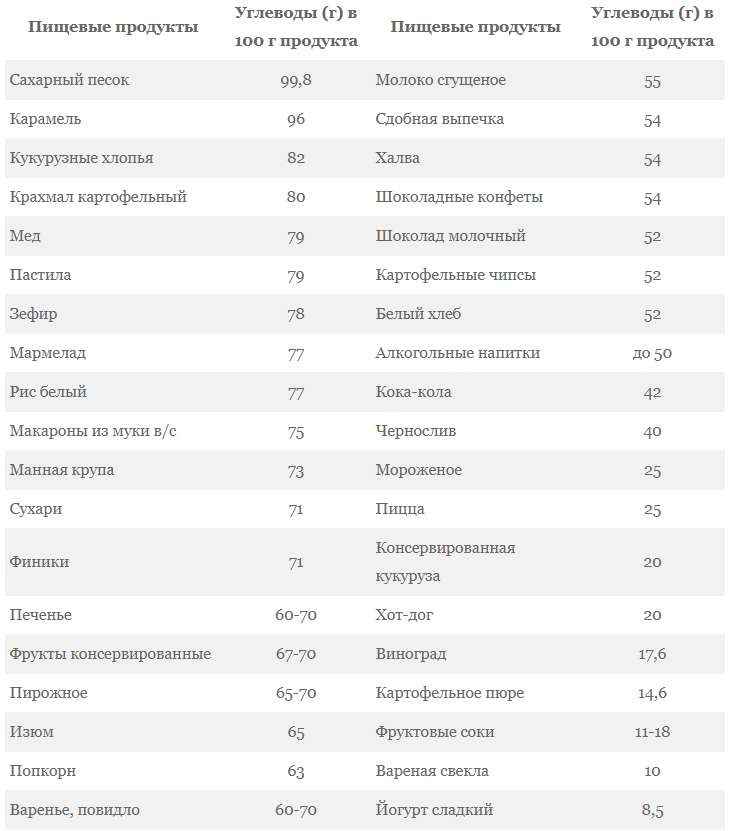
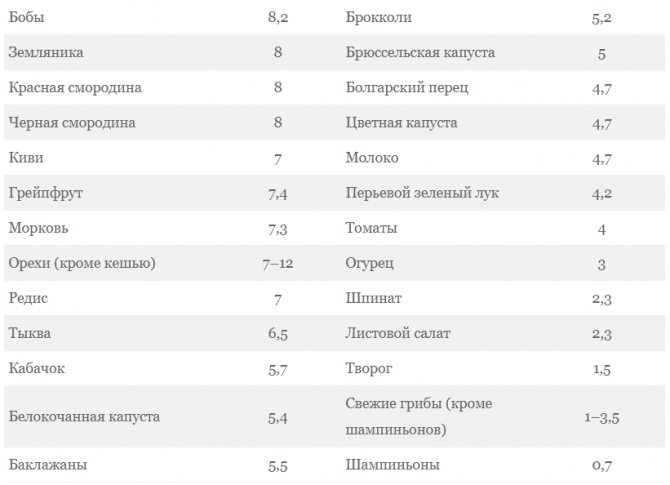
Grocery list
Simple carbohydrates
Complex carbohydrates
Obtaining glucose
Starch hydrolysis
In the presence of acids, starch is hydrolyzed:
(C6H10O5)n + nH2O → nC6H12O6
Synthesis from formaldehyde
The reaction was first studied by A.M. Butlerov. The synthesis takes place in the presence of calcium hydroxide:
6CH2=On → C6H12O6
Photosynthesis
In plants, carbohydrates are formed as a result of the reaction of photosynthesis from CO2 and H2O:
6CO2 + 6H2O → C6H12O6 + 6O2
| Fructose is a structural isomer of glucose. This is a ketone alcohol (ketose): it can also exist in cyclic forms (furanose). |
It contains six carbon atoms, one ketone group and five hydroxyl groups.
| Fructose | α-D-fructose | β-D-fructose |
Fructose is a crystalline substance, highly soluble in water, sweeter than glucose.
It is found freely in honey and fruits.
The chemical properties of fructose are associated with the presence of ketone and five hydroxyl groups.
Hydrogenation of fructose also produces sorbitol.
| Disaccharides are carbohydrates whose molecules consist of two monosaccharide residues connected to each other through the interaction of hydroxyl groups (two hemiacetal or one hemiacetal and one alcohol). |
Daily carbohydrate needs
The daily intake will be different for each person.
On the Internet, some sites claim that the carbohydrate norm is 3–5 g per 1 kg of weight. In reality, everything is more complicated. The norm must be calculated for each person individually .
The need depends on gender, age, weight, activity level , etc. Also, your current goals are of great importance. For example, when losing weight and gaining muscle mass, you need completely different amounts of carbohydrates per day.
As an example, calculations were made for a 30-year-old man and woman of average height and low activity. See table below.
In the case of weight gain, average activity was taken (3 workouts per week).
Based on your gender, weight, and weight goals, you can determine your carbohydrate needs. Of course, the figure is approximate, but the error will not be very large.
| 50 -55 kg | 55-60 kg | 60-65 kg | 65-70 kg | 75-80 kg | 80-85 kg | |
| Men | ||||||
| Weight loss | 140 | 145 | 150 | 155 | 160 | 165 |
| Weight maintenance | 160 | 165 | 170 | 175 | 180 | 185 |
| Muscle gain | 270 | 280 | 290 | 300 | 315 | 325 |
| Women | ||||||
| Weight loss | 110 | 115 | 120 | 125 | 135 | 140 |
| Weight maintenance | 130 | 137 | 145 | 150 | 160 | 165 |
| Muscle gain | 228 | 240 | 245 | 255 | 270 | 280 |
When gaining muscle mass, the body needs a lot of energy. In this case, it is recommended to focus on foods high in carbohydrates. Of course, not forgetting about proteins and fats.
Lack of carbohydrates
A deficiency can cause weakness, fatigue, irritability, and apathy. In addition, muscle tissue and fat reserves, as well as proteins and fats supplied with food, will begin to be used as a source of energy.
Excess carbohydrates
Excessive amounts of them lead to a set of extra pounds .
Abuse of simple carbohydrates can lead to diabetes, arterial hypertension, insulin resistance, cardiovascular diseases and cancer.
Complex carbohydrates , on the contrary, can prevent these and other diseases . Fiber plays a big role here.
When the need increases
Since carbohydrates are the main source of energy, the need for them increases with increased mental and physical activity. If you decide to play sports, gain muscle mass, or your new job involves heavy physical activity, increase the amount of carbohydrate-containing foods.
What have we learned?
From the 10th grade chemistry topic we learned about carbohydrates. These are bioorganic compounds consisting of one or more structural units. One unit or molecule consists of carbonyl and hydroxyl groups. There are monosaccharides, consisting of one molecule, oligosaccharides, including 2-10 molecules, and polysaccharides - long chains of many monosaccharides. Carbohydrates taste sweet and are highly soluble in water (with the exception of polysaccharides). Monosaccharides dissolve in water, oxidize, and interact with copper hydroxide and silver ammonia oxide. Polysaccharides and oligosaccharides undergo hydrolysis. Polysaccharides burn.
Energy function
Carbohydrates are one of the important sources of energy for the body. Energy is released during oxidation under the influence of enzymes. Thus, the breakdown of 1 gram of carbohydrates produces 17.6 kJ of energy. As a result of oxidation and release of energy, water and carbon dioxide are also formed. This process plays a large role in the energy chain of living organisms, since carbohydrates can be broken down to release energy both in the presence of oxygen and without it. And this is very important in case of oxygen deficiency. The sources are glycogen and starch.
Construction function
The structural or construction function of carbohydrates in a cell is that they are building materials. Plant cell walls consist of 20-40% cellulose, and it is known to impart high strength. This is why plant cells maintain their shape well and thus protect intracellular juices.
Chitin is also a building material and is the main component of fungal membranes and the exoskeleton of arthropods. Some oligosugars are present in the cytoplasm of animal cells and form the glycocalyx. Carbohydrate-containing components play the role of a receptor and receive signals from the environment, then transmit information to cells.

Test on the topic
- Question 1 of 10
What are oligosaccharides?
Start test (new tab)
Hall of Fame
To get here, take the test.
-
- Tatiana Barabasheva
7/10
- Yulia Dmitrieva
10/10
- Ksenia Ofuteyan
7/10











