Iron (Fe, Ferrum, ferrum) is one of the microelements (in supplements it can be indicated as Iron) that a person receives from food and water. Included in the category of metals that affect metabolic processes, growth, development, reproductive function, blood composition and quality. Iron is distributed throughout the body as follows:
- Up to 70% is in hemoglobin.
- Up to 20-15% - in the form of depots (reserves) in the liver.
- About 5-10% is in myoglobin (responsible for the respiration of muscle tissue).
- A small part (about 0.1%) is composed of the proteins transferrin, hemosiderin and ferritin.
The role of iron in the body
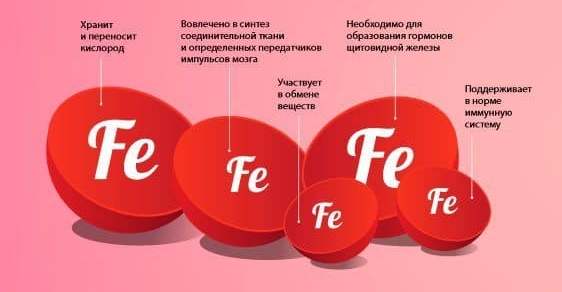
Iron is part of hemoglobin, a component of red blood cells (erythrocytes). These cells carry oxygen and instead take in carbon dioxide and deliver it to the lungs for disposal. Thus, without iron, respiratory processes at the cellular level would simply be impossible.
Other functions of iron in the body:
- Ensuring metabolism. The trace element is involved in the destruction and removal of toxins from the body, the conversion of calories into energy and cholesterol metabolism.
- Protection of the body. Iron is involved in immune processes, activates a person’s own interferon and helps cope with “aggressors” that have entered the body.
- Creation of nerve impulses. Iron not only creates them, but also helps in conducting them along nerve fibers.
- Neutralization of poisons and toxins. This occurs in the liver with the obligatory participation of iron.
The benefits of iron are also invaluable for hair. With its deficiency, hair becomes weak and dull, grows poorly and falls out. This is due to the transport function of iron. When there is little of it, not enough oxygen reaches the hair roots. Deprived of nutrition, they simply cannot grow normally. With prolonged iron deficiency, diffuse alopecia may develop. With it, hair begins to fall out evenly throughout the head.
Iron-containing proteins
The role of iron in the human body is difficult to overestimate. The main function of this element is that it connects proteins that produce hemoglobin. This special protein not only gives blood cells their color, but also performs a vital function - transporting oxygen molecules through the blood to all tissues of the human body.
It is also worth adding that without iron the formation of myoglobin, which is found only in muscle tissue, is impossible. Myoglobin is a very important element, since it is thanks to it that oxygen enters muscle cells, which ensures chemical reactions. These same reactions contribute to muscle contraction in the human body.
The role of iron in the body of all living beings is assessed by the multifaceted spectrum of its functionality and irreplaceable participation in biochemical activity and in the active process of cellular respiration. It is for this reason that even the slightest lack of this element in the body immediately affects a person’s overall well-being, and often even leads to serious problems.
When a person lacks this element, he experiences chronic fatigue, decreased appetite, ulcers and wounds appear - generally speaking, insufficient metal content leads to serious problems with health, well-being and appearance. This essential microelement is also essential for the immune defense of the human body. He acts as the main defender against various “aggressors” - infectious diseases. Considering all the significant and capital processes, the implementation of which is possible only thanks to this microelement, it is always worth monitoring its content in the blood.
Iron norm
To maintain health, men need about 10 mg of iron per day, and women need 15-20 mg. During pregnancy you need even more - 20 mg. The increased dose for women is due to the fact that the level of this element in their body is lower. This is also explained by differences in the hormonal system and monthly iron losses during menstruation.
If we consider iron standards in blood tests, the average values are:
- 9-30 µmol/l – for women with a hemoglobin level of 120-150 g/l and 110-140 g/l during pregnancy;
- 12-31 µmol/l – for men with a hemoglobin level of 130-170 g/l.

Similarities between chlorophyll and hemoglobin
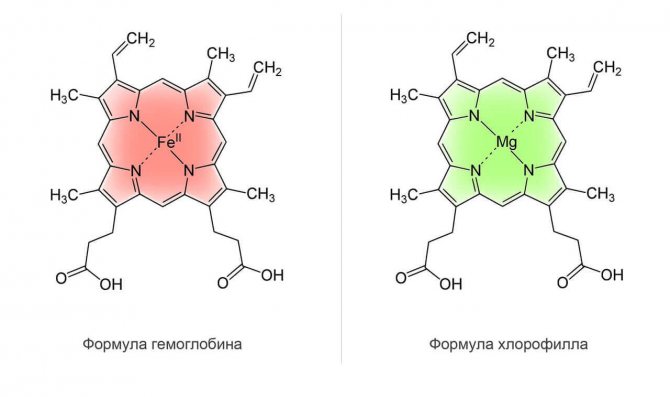
Hemoglobin - hemo (iron component) and globin (protein), in the hemoglobin molecule there are four hemos, more precisely, protomers (they are connected to each other by hydrogen bonds). That is, a certain amount of protein and four iron atoms. Hemoglobin is almost an exact copy of chlorophyll, and chlorophyll is very close to cyanocobalamin. Chlorophyll is part of chloroplasts (the green pigment due to which photosynthesis occurs - the formation of organic substances in the light).
How is chlorophyll different from hemoglobin?
The structure of chlorophyll and hemoglobin are identical, but only in hemoglobin there are 4 iron atoms, and in chlorophyll - 4 magnesium atoms, there is no more difference. This is also related to the difference in color. Therefore, everything that is green (from plants) contains a large amount of chlorophyll.
Symptoms of iron excess and deficiency
To accurately determine the level of the element, a biochemical blood test is prescribed. Excess iron in the body is observed less often than deficiency, but also negatively affects health. This condition is called hemochromatosis. This is indicated by pain in the right hypochondrium and redness of the skin.
Low iron levels are more common than high iron levels. The microelement retains oxygen in red blood cells, so when it is deficient, the body begins to experience hypoxia (oxygen deficiency). In the most severe cases, iron deficiency anemia (popularly known as anemia) may develop. It is especially common in infants and pregnant women.
Symptoms of iron deficiency in the body:
- fatigue and muscle weakness;
- dry hair and brittle nails;
- pale skin (also indicates a lack of hemoglobin);
- tinnitus, headache and dizziness;
- rapid heartbeat and shortness of breath;
- bluish tint to lips and nails.
Even with a deficiency, you may notice difficulties and discomfort when swallowing. Others note that their taste preferences changed in strange ways, for example, they wanted to chew paper or chalk.
In some cases, with iron deficiency, a person feels a loss of strength. Even after sleep he feels tired, and the slightest exertion causes severe shortness of breath. Another person may experience constant colds, which is caused by a decrease in immunity.
On this topic:
How to strengthen the immune system in spring and why it is really necessary (the simplest methods and tips)
Chlorophyll increases hemoglobin
What's the point, you ask, so let's get closer to the main point. In the bone marrow, it is not the formation of red blood cells that occurs, but the assembly of hemoglobin, which is part of the red blood cells. And this happens in a very interesting way, if you simplify it: 4 iron atoms (free-floating in the blood plasma) approach the assembly shop, chlorophyll approaches and 4 magnesium atoms are removed, in whose place iron atoms become, and this process occurs with the mediation of 4 cobalt atoms, thanks cyanocobalamin
(one form of vitamin B12). Thus, our own hemoglobin is formed from chlorophyll. This does not mean that if you have high hemoglobin you cannot drink chlorophyll - such a conclusion is erroneous. Chlorophyll normalizes the level of hemoglobin production if necessary. Otherwise, Chlorophyll will be used for other needs of the body! This is another confirmation that all food chains (networks) work on primary organic biomass and producers (plants) in ecological terms are the basis of the entire food chain. Therefore, it is almost impossible to live a long, high-quality life without plant-based green food.
What foods contain iron?
To get the required amount of iron, you need to eat foods that contain it. For humans, the most valuable iron is heme iron, which contains myoglobin and is present in foods of animal origin. The following products contain it:
- beef liver and other offal;
- beef;
- fish;
- turkey;
- seafood.

Another microelement is obtained from beets, buckwheat, pomegranates and apples. They contain non-heme iron, which is absorbed much worse than heme iron. The leader in content per 100 g here is buckwheat. Moreover, for better absorption it should not be boiled, but steamed overnight by pouring boiling water over it. It is also useful to additionally include foods with vitamin C in your diet, as it helps iron to be absorbed more efficiently.
On this topic:
Benefits of vitamin C for the body | Does it really help against colds and why is it needed in sports?
Hemoglobin function
Since hemoglobin is present in large quantities in red blood cells, its color is bright red (hemoglobin is an iron-containing protein, hence the red color). Hemoglobin is an integral part of the red blood cell and is part of its composition. The task of hemoglobin is to bind, retain and transmit critical oxygen to every cell of our body with the blood stream and take away metabolic products, and most importantly, carbon dioxide and utilize it from the cell.
An increase in the number of red blood cells in the blood indicates either severe dehydration or chronic leukemia. A decrease in the number of red blood cells in the blood from normal indicates anemia, which in the long term leads to serious illnesses.
Microelements. Iron
25.01.2017 Physiological role of microelement.
Iron (Fe) is a trace element that is absorbed by plants in the largest quantities. The content of iron and manganese in plant leaves reaches hundredths of a percent, while the concentration of zinc is expressed in thousandths, and the copper content does not exceed ten thousandths of a percent. That is why iron is sometimes classified as a macronutrient, although in terms of its physiological functions it is a typical micronutrient.
Iron is a functional component, part of plant enzymatic systems. Its role is especially important in oxidative and energy metabolism, as well as in the formation of chlorophyll. Therefore, organic compounds, which include iron, are primarily necessary for plants to carry out biochemical processes that occur during respiration and photosynthesis.
Thus, iron plays one of the key roles in plant biochemistry, because:
· the processes of chlorophyll formation take place with the participation of iron;
· during photosynthesis, organic iron complexes take part in the transfer of electrons;
· non-heme iron-containing proteins take part in the reduction of nitrites and sulfates;
· iron is directly involved in nucleic acid metabolism.
Deficiency symptoms.
Iron deficiency is a problem for many crops. Most types of soil contain enough iron to supply plants. Iron deficiency is observed mainly on carbonate alkaline soils in arid conditions with poor air conditions. The lack of this element negatively affects many physiological processes occurring in plant tissues, leading to a weakening of their growth and development and, as a consequence, a decrease in yield.

A characteristic sign of iron deficiency is chlorosis of the youngest leaves, with leaf veins becoming visible in detail (interveinal chlorosis). With severe iron deficiency, the leaves become yellow to white in color. In this case, fertilizing with iron is no longer useful. Cauliflower leaves are marbled-chlorotic, initially and later they are completely chlorotic. Beetroot has young leaves that are chlorotic with a noticeable red color. In tomato, intense spotty chlorosis appears, occurring at the bases of the apical leaf lobes. The stem and tops also turn yellow.
Symptoms of trace element excess.
Excess iron occurs quite rarely, and the growth of the root system and the entire plant stops. On acidic soils in conditions of excess moisture or on saline soils with low phosphorus and base content, excessive concentrations of iron can have a toxic effect on plants. Dark green leaves, stunted growth, dark brown to purple leaves in some plants (rice bronze disease), damaged leaves and necrotic spots are the most common manifestations of iron toxicity. If for some reason the excess of iron turns out to be very strong, the leaves begin to die and fall off without any visible changes.
With an excess of iron, the absorption of phosphorus and manganese is difficult, so signs of a deficiency of these elements may also appear. However, plants well supplied with nutrients, especially calcium and silica, can tolerate very high concentrations of iron.
Requirement of crops for iron.
Of all the trace metals in plants, the highest content is iron. The normal level of iron in the green leaves of most plants is 100-200 mg/kg of dry matter. Plants such as oats, rice, spinach, and fruit trees are very demanding of iron.

Content of the element in various types of soils.
The concentration of iron in soil solutions ranges from 30-550 µg/l, increasing with increasing acidity (up to 2000 µg/l). The minimum values of soluble iron content are noted at alkaline pH values. This is why acidic soils are rich in inorganic iron to toxic levels, while alkaline, well-aerated soils have low concentrations of soluble iron that cannot meet plant needs. When the pH value is high and the soil is enriched with phosphorus, iron is precipitated in the form of salts and becomes less available to plants. Therefore, the introduction of mineral iron salts into the soil does not eliminate its deficiency in plants, since the iron ions immediately become inaccessible. Excess calcium and manganese in the soil solution also have a negative effect on the physiological activity of iron. Nitrate nutrition limits, and ammonium nutrition enhances the absorption of iron by plants. Soil iron is characterized by a strong affinity for mobile organic complexes and chelates.
Types of iron-containing fertilizers and their use.
Iron sulfate (contains about 20% iron) and iron chelates (10-17% iron) are used as iron-containing fertilizers. Most researchers believe that the chelated form of iron is more effective when applied as a foliar application. However, there are results showing that the inorganic form of iron, provided that adjuvants are used correctly, has the same effect, especially on industrial crops - corn, sorghum.
Iron chelates consist of three components: iron ions, a chelating agent (EDTA, DTPA, EDDHA, amino acids, humic and fulvic acids, citric acid) and sodium or ammonium ions. Different agents retain the iron ion with different strengths at different pH values. At high concentrations of calcium and magnesium, these elements are able to replace the iron ion in the chelate. The possibility and speed of such replacement also depends on the chelating agent.
Fe-EDTA chelate is stable at pH below 6. At pH 6.5, about 50% of the iron becomes inaccessible, so its use does not make sense on alkaline and carbonate soils, where it is easily replaced by calcium. Fe-DTPA chelate is more stable (up to pH 7.0), iron in it is not replaced by calcium. Fe-EDDHA chelate is the most stable (works in a pH range up to 11), but it is the most expensive.
Pre-sowing seed treatment.
The iron concentration for many agricultural plants when treating seeds with inorganic iron salts is 1-2.5%. However, much more often iron is included in micronutrient complexes for seed treatment. For crops sensitive to iron deficiency, Fe-EDDHA chelate is effective for seed treatment. It is a dry powder that is mixed with water and the seeds are treated with this solution before sowing. The best results are achieved by combining pre-sowing treatment and foliar application of Fe-EDDHA during the growing season of the crop.
Application of iron-containing fertilizers to the soil.
Typically, inorganic forms are not used for soil application due to the rapid fixation of iron in the soil, although this is practiced for iron-poor soils in some regions of the world. In American studies, row application of 80 kg of iron sulfate per hectare increased corn yield by 15%.
For soil application, iron chelates with EDDHA and EDDHMA are the best choice, providing product stability and iron availability in very alkaline soils. Iron chelates with HEDTA, DTPA and EDTA can also be used for soil application on soils with pH>6. Soil application of iron chelates is an effective way to deliver this element to the plant, but historically the cost of such treatments has been prohibitive. Currently, a number of preparations have been developed for seed treatment that contain Fe-chelates and are affordable. Thus, iron enters the soil and is immediately used by seedlings.

Foliar feeding.
To prevent and treat chlorosis by foliar feeding, both chelated forms of iron and inorganic forms (ferrous sulfate, ferrous nitrate) are effective. For foliar applications, EDTA chelates are used in the vast majority of cases, including for iron. For very hard water, Fe-DTPA can be used. Concentration of iron in solution according to d.v. – 0.5 mg/l.
The concentration of iron is of great importance - absorption by leaves increases with a decrease in the concentration of this element in the solution.
Another method of adding iron, which is still quite exotic for our conditions, is injection of solutions of microelements into fruit trees. Thus, the spring and autumn introduction of a 1% solution of iron sulfate into apple trees made it possible to eliminate chlorosis caused by iron deficiency for 3-4 years and was very inexpensive. Currently, methods are being developed to reduce the infection of trees when various drugs are injected into them, which is the main obstacle to the spread of this method.
Iron chelates in liquid ready-made fertilizers are destroyed by exposure to light; therefore, it is recommended to store such preparations in a dark place.