Micronutrient-rich foods
To ensure a sufficient supply of sulfur to the human body, one must have a balanced diet, which will include both sufficient amounts of protein and products of animal and plant origin. Also don’t forget vitamin B and amino acids. If you wish, you can consult a nutritionist to create a proper diet.
| Product | Sulfur content per 100 g | Percentage of daily requirement |
| Milk (powdered, low-fat) | 339 mg | 35% |
| Milk (powdered, 35%) | 261 mg | 27% |
| Soybean (grain) | 245 mg | 25% |
| Cottage cheese | 222 mg | 23 % |
| Chickpeas | 200 mg | 21 % |
| Chicken egg (white) | 188 mg | 20 % |
| Cottage cheese 9% | 182 mg | 19% |
| Almond | 200 mg | 19% |
| Chicken egg | 178 mg | 19 % |
| Peas | 172 mg | 18 % |
| Chicken egg (yolk) | 172 mg | 18 % |
| Lentils | 163 mg | 16 % |
| Beans | 160 mg | 17 % |
| Quail egg | 125 mg | 13 % |
| Walnut | 102 mg | 11 % |
| Cabbage | 200 mg | 19 % |
| Poppy | 640 mg | 63 % |
| Beef liver | 239 mg | 22 % |
| Mung beans | 238.6 mg | 24 % |
| Hot smoked fish | 233 mg | 24 % |
| Rabbit meat | 225 mg | 23 % |
| Salmon | 225 mg | 23 % |
| Brynza | 221 mg | 21 % |
Interaction with other substances
The “opponents” of sulfur are Selenium, Barium, and also heavy metals – molybdenum and lead, Mo.
For better absorption of sulfur, the following products will help:
- fish;
- honey;
- bran;
- pumpkin;
- oatmeal;
- peas;
- poultry and rabbit meat;
- Rye bread;
- buckwheat.
Sulfur: benefits and harms, instructions for use, role in the body
The benefits and harms of sulfur lie in the effects of sulfur-containing drugs on the body.
In order to understand their effect on health and learn how to use it, it will be useful to learn about the role of this substance for our body.
What is sulfur and its role in the body
Sulfur, S (full name sulfur), is a macroelement assigned atomic number 16 in the periodic table.
This substance, known for its fetid odor in compounds and flammable properties, plays an important role in humans, being part of amino acids such as methonine, cystine, vitamins (for example, thiamine), hormones and enzymes (for example, insulin).
In the human body, the proportion of sulfur is 0.25% of the total mass.
Sulfur itself is not toxic, but compounds of the element with other chemical components, such as hydrogen sulfide, are poisonous.
As part of the body's compounds, sulfur has benefits for the growth of hair, nails, skin, and also in protecting the body from aging.
Application of sulfur
Sulfur has been used to treat humans since ancient times. Modern medicine uses a number of medicines containing this element and its compounds. For example:
- natural hydrogen sulfide baths have a beneficial effect on the body;
- sodium thiosulfate solution is used to treat scabies, neuralgia, arthritis;
- streptocide and phthalazole serve as antiseptic drugs.
Sulfur is used to produce medicinal soap, which has an antiseptic and drying effect. Purified, or medical, sulfur is used to fight parasites, intestinal diseases and frequent constipation.
Sulfur is used to treat eczema, furunculosis and demodicosis. Hair and anti-dandruff masks are made from it: the element acts as a hair growth stimulator and also eliminates oiliness.
Advice! Doctors recommend therapy using sulfur two to three times a year.
Indications for use
Doctors prescribe treatment for sulfur when it is deficient in the body. Medicines made on the basis of the element have an antiseptic and antibacterial effect; they are used for the treatment of scabies, fungal diseases, and juvenile acne.
The healing properties of sulfur are recommended for use by people suffering from rheumatism and osteoarthritis. The main thing is to follow the recommendations for use, since an overdose of the drug can cause harmful intoxication.
How to use sulfur
Sulfur-containing drugs are taken simultaneously with food.
You should only buy purified sulfur from the pharmacy.
Feed sulfur preparations for animals are not intended for human consumption.
The optimal course of treatment would be 1 month. If there is no expected benefit, you should stop taking it until you consult your doctor.
Important! Sulfur is used as prescribed by a doctor in the recommended dosage. Self-administration and prescription of the drug is not recommended
Sulfur powder for oral administration
Powder for internal use is prescribed for chronic diseases:
- polyarthritis;
- sciatica;
- haemorrhoids.
Sulfur for oral administration is purified and comes in tightly sealed jars. Taking purified sulfur is also prescribed for the treatment of enterobiasis.
Medical purified sulfur, or sulfuric anhydride, is beneficial in cases of:
- restoration of the body's defenses;
- for expectorant purposes: hydrogen sulfide is absorbed through the intestines and enters the lungs through the blood;
- for constipation, purified sublimated sulfur is prescribed;
- to enhance the body’s ability to neutralize weak poisons.
When taking sulfur, you can drink enterosorbents if you wish: they will reduce the harm of frequent gas formation that the drug can cause.
Products containing sulfur
Many organic products contain this useful element. Their daily use can bring benefits comparable to taking sulfur powder orally:
- bulb onions;
- peas;
- a pineapple;
- zucchini;
- tomatoes;
- turnip;
- watermelon;
- nuts.
Quail and chicken eggs contain the largest amount of the element.
There are also useful organic sulfur supplements on the market, such as chewable Siberian larch gum.
Daily intake of sulfur
The consumption rate for the benefit of the body is from 500 mg to 1 g per day. The dose for athletes due to increased physical activity is increased to 3 g per day, and mineral water containing sulfur is also prescribed in the diet.
Symptoms of lack of sulfur in the body
If the body lacks an element, a person’s immunity decreases, which is manifested by a decrease in vitality, lethargy and fatigue. Disturbances in the functioning of the body are reflected in appearance.
Receipt
Sulfur is one of the most abundant elements on earth. Large sulfur deposits are located in the USA, Canada, CIS countries, China, Mexico, Saudi Arabia and Poland. Also present as various ions from soluble compounds in seas and oceans or in natural sources. One of the most famous sulfur springs is located in Karlovy Vary.
Previously, only solid minerals were the source of sulfur: 3.5 million tons were mined annually using the Frasch process developed by Hermann Frasch, mainly in the USA and Poland. The largest share was sulfur extracted from sulfide ores. Today, sulfur is produced in large quantities as a waste product from oil desulfurization using the Claus process. Fossil fuels such as coal and oil contain large amounts of sulfur. Therefore, petroleum fractions that are used to generate energy are desulfurized before further processing.
Since sulfur deposits do not satisfy modern needs for this raw material, sulfur is extracted from other natural compounds and ores. Sulfur, which is produced as a toxic waste product from many combustion processes, can be recovered through recovery. For example, hydrogen sulfide is produced during the processing of natural or coke oven gas. It is a highly toxic gas that can be converted to water and sulfur using oxygen and a catalyst.
About half of the total volume is obtained from solid sulfur and subsequent purification by distillation. A pipe 150 to 800 meters long under pressure supplies hot water to sulfur-containing rocks. The underground sulfur is melted and then transported upward by hot compressed air. The resulting sulfur purity ranges from 99.5 to 99.8%. One well can produce up to 300 tons of sulfur per day. The other major portion comes from the desulfurization of crude oil and natural gas using the Claus process. One third of the hydrogen sulfide H2S contained in natural gas or coke oven gases is burned with oxygen in the combustion chamber to form sulfur dioxide.
Sulfur can also be obtained by roasting pyrite. When the ore is heated, sulfur dioxide is produced, which is then reduced with carbon or coke.
Instructions for taking sulfur
Sulfur is very useful for the human body, so to use the mineral for therapy you need to buy it in pharmacies. Purified or precipitated sulfur is intended for internal use by humans. There will be no adverse reactions when using them. The maximum that can happen is increased gas formation from purified sulfur.
To use sulfur internally, as a food additive, as mentioned above, it must be purchased purified in pharmacies. Sulfur powder should be taken no more than 0.45 g per day. The substance can be divided into 3 parts, and also washed down with water during meals.

Sulfur can be added to dishes and drinks - this method is applicable to small children, as if the powder is accidentally inhaled, a choking attack may occur. Sulfur powder is an excellent remedy for getting rid of acne and inflammation on the skin because it has a drying property. You should take no more than 1 g of the mineral per day; doctors advise sticking to a dosage of 0.5 g.
When using ointment, it should be applied in a very thin layer only to clean areas of the skin, previously washed with soap, so as not to disturb the fatty film. If it is damaged, air exchange with the environment will be disrupted. The action of sulfur ointment is aimed at restoring the epidermis. The substance is applied for a day, after which it is washed off with plain water.
Brewer's yeast with sulfur is used to treat acne, furunculosis and hair loss. The substance is taken orally in the form of tablets or granules. Masks for the face and scalp are prepared using sulfur brewer's yeast.
Chewable sulfur
Chewable sulfur is a larch resin that has a positive effect on the oral cavity and the entire body as a whole. The properties of this type of mineral will help get rid of premature tooth decay and loss. In case of inflammation of the gums, the drug must be kneaded like plasticine and begin to chew, and then placed on the “damaged” area.
It is strictly forbidden to use chewing resin for periodontal disease, as it promotes a strong flow of blood to the gums, thereby contributing to the development of the disease. Doctors recommend chewing sulfur in stressful situations and for people who live in unfavorable environmental conditions, for example, in conditions of radiation.
Vitamin-mineral complexes with the inclusion of sulfur are in great demand in pharmacies; when they are used, skin cells are renewed, collagen synthesis is enhanced, and hair growth improves.
Sulfur is an essential element for young, beautiful skin and healthy, silky hair. It is part of the amino acids responsible for the processes of collagen synthesis and keratin activity.
With the complex use of the mineral, skin turgor is strengthened, facial wrinkles are smoothed out, and the process of skin cell renewal begins. Sulfur helps reduce inflammation, cleanse pores, and block pathogenic flora.
When treating shingles, sulfur is often used in combination with glycerin. This tandem will completely relieve pain during illness. Glycerin and sulfur are mixed in proportions 2:1. The first has a moisturizing effect on the skin, and the second suppresses the spread of the virus and relieves inflammation.
When you start using such a compress, you may feel a slight itching - this means that the skin is healing successfully. For greater effect, you can add boric acid to the mixture, which promotes an antibacterial effect and prevents other bacteria from being introduced into the wound.
Sulfur has its own derivatives, which are often used in medicine to block symptoms or completely eliminate them:
- sulfur color - used for diseases of the scalp (scabies, eczema, seborrhea);
- sulfur milk is an antiparasitic agent that acts on certain microorganisms and harmful bacteria.
- purified sulfur - used as a laxative for diarrhea, it should be taken several times a day.
Hydrogen sulfide baths are very common in medicine. They are prescribed to people who have problems with the nervous system, spine, or suffer from hypertension. Hydrogen sulfide baths also have a positive effect on patients with varicose veins, diabetes and slow metabolism. This procedure is also common in the field of urology and gynecology.
- with heart disease;
- kidney;
- liver;
- patients with ischemia and oncology.

Hydrogen sulfide baths are also prohibited if patients have:
- bronchial asthma;
- chronic stomach ulcer;
- oncology;
- hyperthyroidism.
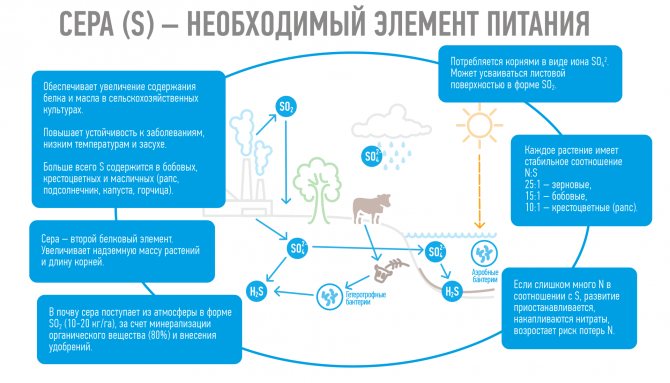
"Queen of Mesoelements"
When people talk about mineral nutrition for plants, they most often mean the supply of nitrogen, phosphorus and potassium. But we must not forget about the fourth most important mesoelement - sulfur.
If there is not enough sulfur...
Sulfur is an amazing macronutrient. The plant does not need so much of it, but if there is a shortage of it, many vital processes freeze. And all because sulfur takes part in nitrogen and carbohydrate metabolism, in the process of respiration and fat synthesis. Sulfur is part of proteins and vitamins. It promotes the accumulation of starch and sugars in the plant. Thanks to it, the growth and absorption activity of the root system is enhanced, as a result of which plants better absorb nitrogen and phosphorus.
In addition to the fact that sulfur is simply necessary for the normal growth and development of the plant, it improves the quality of products - it helps to increase the proportion of gluten in wheat, the oil content in sunflower, soybeans, and rapeseed. Not only their taste improves, but also their nutritional value for animals and humans. Sulfur is responsible for blood clotting, helps the body fight harmful bacteria, protects against radiation exposure and slows down aging. Recently, due to sulfur deficiency in fields and greenhouses, there is little of this element in plant foods.

You can tell that a plant needs sulfur by its appearance. Lack of sulfur leads to a decrease in photosynthesis by 40%. As a result, the leaves become smaller and lighter. First, the veins of young leaves turn yellow, and then chlorosis develops over the entire surface. Plants from the mustard family become reddish-purple over time. The petioles are stretched. The stems do not grow wide. Plants develop worse.
Chlorosis on young leaves and growing points due to sulfur deficiency is explained by the fact that this element cannot move from the lower tiers to young leaves, like nitrogen. Therefore, if, with a deficiency of the main protein element, the old lower leaves first suffer, then with sulfur starvation, first of all, the “youth” are affected. And if, due to a lack of nitrogen, the lower leaves fall off, then sulfur deficiency only leads to paleness and yellowing, but not to the falling of leaves.
Chemical tissue analysis will help determine exactly what the plant lacks. Moreover, fertilizing with sulfur-containing fertilizers, carried out after the appearance of signs of sulfur deficiency, may turn out to be late and will not help restore the growth of crops.
In addition, a lack of sulfur may not appear at all on the appearance of plants, but at the same time reduce the quality of the crop. After all, the activity of proteins and enzymes in the tissues of leaves and seeds depends on the level of sulfur nutrition. This macronutrient increases the fat content of oilseeds. It maintains the three-dimensional structure of proteins by forming disulfide bridges. In wheat proteins, bonds between sulfur atoms give gluten elastic properties, which is important when baking bread. Dough made with high-sulfur flour works better and produces fluffier loaves. The low sulfur content in wheat and, accordingly, in flour worsens the appearance of baked goods baked from it.
All crops – according to need
Most proteins contain sulfur in their composition. On average, in the structure of protein, there is 1 part sulfur for 15 parts of nitrogen. However, depending on the crop family, this ratio may vary. For grains, the N:S ratio will be 25:1, that is, they need much more nitrogen. Legumes fall into an average ratio of 15:1. Cruciferous plants need sulfur in a ratio of 10:1, so it is impossible to grow high-quality cabbage, broccoli or rapeseed without fertilizing with sulfur-containing fertilizers.
The need of plants for sulfur also depends on the phase of the growing season. In rapeseeds, the N:S ratio can be 6:1, so that during the flowering and seed formation phase it absorbs sulfur most intensively. Corn absorbs this element at a constant rate, and half of the sulfur accumulates in the grain. The maximum sulfur content in wheat was recorded in the milky ripeness phase of the grain. Then the crop begins to lose reserves.
If wheat or sugar beets require relatively little sulfur, this does not mean that its deficiency can be ignored. The fact is that macronutrients are interdependent, and a lack of one leads to problems with the absorption of the other. For example, in legumes, a lack of sulfur manifests itself in a decrease in the number of nodules on the roots of plants. This, in turn, leads to a decrease in atmospheric nitrogen fixation.
Nitrogen is also less absorbed from the soil if such an important component for the construction of proteins as sulfur is missing. It turns out that in sulfur-deficient fields fed with nitrogen fertilizers, nitrogen is simply washed away. Damage is dealt in all directions. The farmer wastes money because the yield does not increase. The environment is suffering. Nitrates accumulate in the final agricultural products, which clearly does not please consumers.
In Germany, as researchers have calculated, up to 300 million kg of nitrogen is lost annually due to a lack of sulfur in the soil, or about 10% of the nitrogen fertilizers used in the country.
Based on the average ratio of nitrogen and sulfur in proteins, scientists have derived a formula that every kilogram of sulfur lost by plants is a potential cause of loss of 15 kg of nitrogen. And the world's leading fertilizer manufacturer, taking this rule into account, has developed sulfur-containing complex fertilizers, such as sulfoammophos, a UAN + S mixture, recently launched into production in Krasnodar.
The specific role of the “taste and smell molecule”
Unlike nitrogen, sulfur is part of only “selected” amino acids: cysteine, cystine and methionine. Cysteine is the simplest structure from which plants build many other sulfur-containing organic compounds. Cystine, for example, is two cysteine molecules joined together. Methionine is an essential amino acid that is not synthesized in the human body.
The three named amino acids contain up to 90% of the sulfur found in the plant. The remaining 10% comes from other compounds. Sulfur is found in vitamins and coenzymes such as thiamine (B1), biotin (B7), coenzyme A, glutathione, lipoic acid. It is part of enzymes, including those responsible for plant respiration, for example, dehydrogenase. Sulfur is essential in vegetable oils.
Sulfur is also contained in specific compounds that give plants a special taste and aroma. Rapeseed and mustard produce glucosinolates, which is why they become bitter and pungent. Garlic and onions produce sulfur-containing alliins. The characteristic taste and smell of onions and garlic are due to these volatile compounds. If there is enough sulfur in the soil, the pungency of mustard and the aroma of garlic intensify.
By the way, sulfur-containing aromatic compounds increase the resistance of plants to damage by pests and stress caused by unfavorable external factors.
From soil and air
In the soil, sulfur is found in organic matter in the form of easily soluble sulfates. This is the main source of sulfur nutrition for plants. Sulfuric acid anions (SO42-) are absorbed through root hairs. Then, with the participation of ATP and magnesium, it quickly turns into organic form. Through a chain of transformations, sulfate is reduced and goes to the construction of cysteine in the chloroplasts of leaves. Cysteine also produces the more complex compounds cystine, methionine and glutathione. The latter is involved in the movement of sulfur throughout the plant.
Organic compounds created on the basis of sulfur move through the phloem to places of active protein synthesis - the tips of the roots, the tops of the stems, to the fruits and grains. Subsequently, sulfur can be oxidized again to the sulfate anion, but in this form it becomes immobile. It turns out that in young organs this macroelement is found mainly in a reduced form, and in old organs it is in an oxidized form.
Sulfate ion promotes better absorption of nitrate and phosphate ions. There is no competition between them. All of them are involved in the construction of cells, like bricks, and in metabolism.
In addition, plant leaves absorb sulfur dioxide (SO2) from the atmosphere. We are talking about small quantities - about 1 kg of sulfur per hectare per year.
The higher the technology, the less sulfur
Until recently, no one thought that sulfur deficiency could occur on agricultural land. Free sulfur and its compounds, sulfates and sulfides, are widespread in nature. However, due to the widespread increase in agricultural yields, the removal of sulfur from products has increased and now ranges from 10 to 30 kg S/ha. The specific figure depends on the crop being cultivated and the level of yield. For some plant species from the genus Brassica (Cabbage), sulfur absorption can reach 70 kg S/ha.
At the same time, sources of sulfur began to dry up. Previously, it was found in abundance in man-made emissions. Sulfur dioxide entered the soil from the atmosphere with dust and precipitation. However, in recent years, requirements from environmentalists and environmental government agencies have become stricter, and emissions from industrial facilities have sharply decreased. Accordingly, the flow of sulfur into the soil decreased.
Organic fertilizers were an important source of this macronutrient. Nowadays livestock farming is in decline, and they are almost never used.
The third source of sulfur – phosphorus fertilizers – is also becoming scarce. Previously, the only phosphorus fertilizer used was superphosphate. It is produced using sulfuric acid and contains about 12% sulfur. But with the development of technology, chemical companies switched to phosphate fertilizers with low impurity content. A side effect was a reduction in the dose of sulfur.
No-till technology has contributed to the depletion of soils in terms of sulfur. In the first time after the transition to “zero,” organic matter accumulates, and the level of mineralization decreases. Excess sulfur, alas, is not stored for a long time: it enters into chemical reactions and is washed away. If irrigation is carried out with water purified from sulfates, then a sulfur deficiency will be inevitable.
Initially, sandy and eroded soils are poor in sulfur and have lost a significant part of the top fertile layer. The fact is that up to 70% of soil sulfur is found in humus. For every 77 kg of organic matter there is 0.454 kg of sulfur. It is mineralized by microorganisms to hydrogen sulfide H2S, which, under the influence of oxygen, turns into sulfate ion and is absorbed by plants.
It would seem that chernozems rich in humus should not be affected by the problem of sulfur deficiency. However, this phenomenon has also been recorded in the south of Russia. According to a comprehensive agrochemical survey of agricultural lands in the Stavropol and Krasnodar territories, Rostov and Volgograd regions, more than half of the arable land is poor in gray soil.
In Russia as a whole, only 10% of arable lands contain a sufficient percentage of sulfur – more than 12 mg/kg. The average level is maintained on 30% of the land. The lion's share is land with low (less than 6 mg/kg) sulfur content. Approximately three-quarters of Russia's arable land requires the use of sulfur-containing fertilizers.
There is a solution: sulfoammophos!
Fertilizers containing both the “queen of mesoelements” and the “king of agriculture” – nitrogen, can quickly and effectively provide plants with sulfur. At the same time, for the carbonate soils of the Southern and North Caucasus federal districts, the presence of another important macroelement in fertilizers is important - water-soluble phosphorus.
The optimal combination of these elements for agricultural crops has been selected in different brands of sulfoammophos, which is produced. In the products of Nevinnomyssky Azot, the N:P ratio is 20:20, and the sulfur content ranges from 8 to 14%. This fertilizer also contains calcium and magnesium (0.5% each).
Sulfoammophos contains sulfur in sulfate form - the most accessible for absorption by plants. It is used both for the main application during sowing and for plant nutrition. It works most effectively on crops with an increased need for sulfur, for example, rapeseed. Winter grains in the early stages of development, potatoes, and vegetables also respond well to it.
Tested in Stavropol region
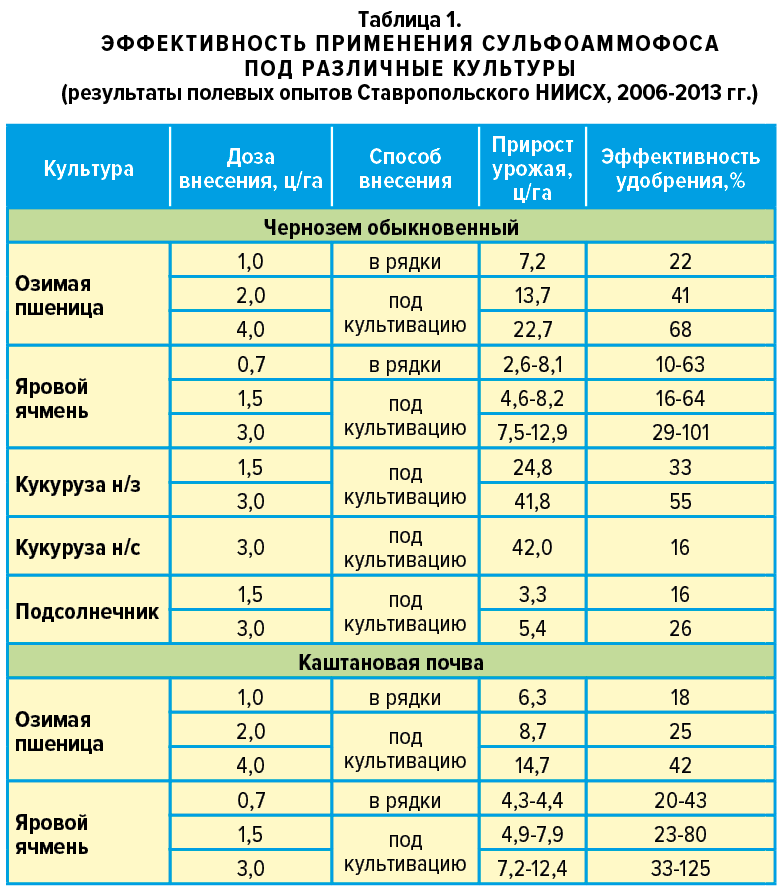
The effectiveness of sulfoammophos on different crops was studied by the Stavropol Research Institute of Agriculture for several years in a row, from 2006 to 2013.
The main grain crop, winter wheat, when applying sulfur-containing fertilizer, showed a yield of 7-23 c/ha higher on chernozems and 4-15 c/ha on chestnut soils. The difference is explained by moisture conditions, agrochemical state of the soil, doses and methods of sulfur nutrition.
Sulfoammophos contributed to an increase in productive tillering by 22-36%. The weight of grain from one ear increased by 6-16%. The gluten content in grain increased to 4%. If we talk about the economy, then a ruble of costs was returned in 3-5 rubles on black soil and 2-4 rubles on chestnut soil.

Spring grain crops consume fewer nutrients during their shorter growing season. They have worse bushiness and development of the root system. Therefore, to increase their productivity, intensive nutrition is required. Sulfoammophos on spring barley at a dose of 3 c/ha, as established by Stavropol scientists, gives an increase in yield of about 12 c/ha. Not only the mass of 1000 grains increases by approximately 3-5 g, but also the number of grains in the ear. The economic benefit ranges from 2.5 to 7 rubles per 1 ruble of the cost of purchasing and applying sulfoammophos.
Corn is the leader in nutrient consumption among all grain crops. It needs a lot of macronutrients throughout the growing season, so basic fertilization is preferable. In addition to nitrogen, phosphorus and potassium, corn needs to be fed with sulfur. By increasing the dose of sulfoammophos by 2 times (from 1.5 to 3 c/ha), you can achieve a proportional increase in yield (from 24.8 to 41.8 c/ha). The green mass, the number of ears and the weight of grain on them are multiplied. Payback 1 rub. costs reach 6 rubles.
Sunflower, being an oilseed crop, especially needs sulfur-containing fertilizers, although it responds less to their application. Sulfoammophos increases sunflower yield by 3-5.4 c/ha and increases the fat content in seeds by 4.8%. This effect is caused by an increase in the diameter of the basket, in which 14-20% more seeds are formed. The weight of 1000 seeds also increases.
In 2015, the Stavropol Research Institute of Agriculture conducted another series of experiments on the effectiveness of sulfur nutrition in the Stavropol Territory and Rostov Region. The control plots differed from the studied ones only in the absence of sulfur in fertilizers.
The year's conditions were favorable in terms of moisture, and the application of 2.6 c/ha of sulfoammophos (N 52P52) provided a significant increase in winter crop yields. Rapeseed yield was almost twice (48%) higher than the control, giving an additional 13.4 c/ha. Barley added 16.7 c/ha, or 27%. The wheat yield turned out to be higher by 21 c/ha, which was 37% compared to the control.
The yield of rapeseed seeds per plant increased by 63.2%. In the crops of winter barley and winter wheat, the number of productive stems increased by 38.9 and 33.3%, respectively. The mass fraction of raw gluten in winter wheat grain increased to 26%, that is, almost 10% compared to the control. The payback of 1 ruble of costs for rapeseed, barley and wheat was 2.78; 1.53 and 2.36 rubles.
When comparing the effectiveness of sulfoammophos before sowing rapeseed with the effectiveness of the scheme “ammophos before sowing and ammonium nitrate in early spring feeding,” it turned out that sulfoammophos reduces costs. Yes, it costs more. Production costs per 1 hectare of rapeseed last year were 428 rubles. more. But then the increase in yield from the use of sulfoammophos reduced the cost of production of 1 ton of seeds by 857 rubles. Productivity increased by almost 6 c/ha. The highest economic efficiency in 2015 was achieved when using sulfoammophos at a dosage of 2.6 c/ha.
On winter wheat, Stavropol farmers also often use ammophos before sowing and ammonium nitrate in the spring. But sulfoammophos also benefits from this crop. Despite the fact that its application in 2015 required 428 rubles/ha more than with the traditional nutrition scheme, the cost of 1 ton of grain decreased by 192 rubles, and gross profit and production profitability increased by 2.1 and 12.3 % respectively.
The hard-working “duet” of UAN + sulfur
Speaking about the advantages of sulfoammophos, do not forget that this is a granular fertilizer that requires moisture to dissolve. In summer in the south of Russia its use is irrational. How, then, can we feed agricultural crops that lack sulfur?
MCC EuroChem gave an excellent answer to this question in 2021. Agrocenter EuroChem-Krasnodar began producing a new fertilizer, UAN+ sulfur. It combines ammonium sulfate and all the advantages of a urea-ammonium mixture, including three forms of nitrogen and a liquid form that does not require additional moisture for dissolution.
Since sulfate forms of sulfur are available to plants, UAN+ sulfur can be used during the period of greatest nutrient consumption - during ear filling, cob and head formation.
Summarizing all of the above, we can add that the use of sulfur-containing fertilizers, rich in other macroelements, gives a synergistic effect. Productivity increases with a parallel improvement in product quality: wheat grain is enriched with gluten, oilseeds become saturated with fats.
The beneficial effect of macroelements when used in combination is also noted by the head of the soil laboratory of the Stavropol Research Institute of Agriculture, Nadezhda Shapovalova, under whose leadership the described experiments were carried out. “Fertilizers that contain both nitrogen and sulfur have a higher effect due to the synergy of these elements. They complement each other during metabolism in plants,” she noted. “In our experiments, we observed this phenomenon in the form of a more intensive increase in yield and quality against the background of equalizing the amount of nitrogen and phosphorus.”

Oversupply
Sulfur is very useful for the human body, but in nature there are also mineral compounds that are highly toxic - these are hydrogen sulfide, carbon disulfide and various sulfur oxides. They are produced in hazardous industries, fires or chemical warehouses when environmental conditions are violated.
Some researchers believe that the use of sulfur-containing preservatives contributes to the development of bronchial asthma.
To avoid high levels of sulfur in the human body, it is recommended to consume raw, fatty poultry meat and fresh eggs in your diet.
Being in nature
Sulfur is found in the earth's crust with a proportion of 0.048% (15th element in terms of frequency of distribution). Huge deposits are located in Sicily, Poland, Iraq, Iran, Louisiana, Texas and Mexico. Sulfur is common in sulfide minerals such as pyrite (FeS2), chalcopyrite (CuFeS2), galena (PbS) and sphalerite (ZnS). Most metals (especially heavy metals) occur in nature as poorly soluble sulfides.
Important sources of sulfur are fossil fuels such as oil, natural gas and coal. In particular, natural gas contains relatively large amounts of hydrogen sulfide (H2S).
Important minerals containing sulfur compounds are gypsum (calcium sulfate), pyrite and marcasite (iron sulfide), copper colored gravel ( copper sulfide), galena (lead sulfide), zinc blende (zinc sulfide) or cinnabar (mercury sulfide). Large amounts of sulfur are also found in fossil fuels, oil and coal. Natural gas is often contaminated with hydrogen sulfide.
Medicines made from sulfur
In its pure form, sulfur is sold in pharmacies in the form of sulfur powder, but it is rarely recommended for severe skin diseases.

More often, sulfur ointment is used for dermatological problems. In addition to the main substance, it contains water and petroleum jelly, which form an emulsion. The ointment has an antimicrobial effect and restores damaged skin epidermis. In addition, it is recommended to combat acne and pimples. It also has an antifungal effect.
Sulfur soap is used for medicinal and cosmetic purposes. Soap with a sulfur content of 10% or more has a healing effect on the skin. And soap containing about 3% of the substance is used for prevention, cleansing the skin, fighting acne, etc.

Sulfur is included in medicinal plasters for calluses.
Used as part of laxatives to treat constipation. Natural mineral water often contains dissolved hydrogen sulfide.
Hydrogen sulfide water is used in various diets and health procedures; hydrogen sulfide baths are beneficial for the skin. They are also recommended for joint diseases - arthritis, rheumatism, etc. Hydrogen sulfide water has a healing effect on the musculoskeletal system and lowers blood pressure.
Finally, sulfur is included in many dietary supplements, along with other macro- and microelements.
Where to buy sulfur, which one is suitable, price?
You should buy sulfur only in pharmacies, including veterinary ones. The mineral is sold in various forms; to determine which drug a person needs, it is better to first consult with your doctor or pharmacist, who will tell you why and what is needed.

For example, purified sulfur or sulfur powder must be taken orally; sulfur ointment – used for scalp diseases; in the form of granules and tablets - for the treatment of acne and other inflammations. The average price of sulfur per 1 kg is 80 rubles.
Sulfur is an important macronutrient for the human body that is present throughout life. Without it, many life processes are impossible. Human health and appearance directly depend on sulfur. It’s not for nothing that people have always welcomed the consumption of animal products with a high sulfur content, because it’s not for nothing that they call it “the mineral of beauty.”
Article design: Oleg Lozinsky
History - brief information
The first mentions of the use of sulfur date back long before the birth of Jesus Christ. So, even the ancient Greek poet Homer, who lived in 9-8 centuries. BC. described the use of sulfur in warfare - as incendiary mixtures. The Chinese also used sulfur to make gunpowder and other pyrotechnic flammable mixtures, and already in the 8th century after Christmas.
Sulfur was also used by ancient priests for various religious rituals.
In the second millennium, S found widespread use in alchemy; it was also considered one of the essential bases of any metal.
The natural nature of sulfur and its properties through various experiments and combustion had already been established by the French naturalist chemist Antoine Laurent Lavoisier (1743-1794).
Useful properties of sulfur
Sulfur has many beneficial properties:
takes part in tissue metabolism; Sulfur is important for the human body as it performs a number of functions.
is part of amino acids; maintains oxygen balance in the human body. promotes active activity of the nervous system; maintains normal sugar balance; is one of the powerful antiallergens; Great for boosting immunity. Humans need sulfur no less than vitamin D; participates in the proper formation of bones (which is especially important during the development of a child); improves the performance of ligaments and joints; responsible for collagen synthesis; has a healing effect; removes toxins and waste from the liver; synthesizes melanin and keratin; participates in the formation of vitamins, including biotin and lipoic acid; prevents cramps and joint pain.
Advantages and disadvantages
If you carefully study the reviews of consumers who managed to test the effects of the drugs on their sites, then in these comments you can find both positive and negative responses. After all, the product has a number of disadvantages.
Pros and cons of colloidal suspension
Advantages Disadvantages has an effective, long-lasting effect; the drug has acaricidal properties; mixing of the working mass with other substances is allowed; sulfur vapors do not harm plants; can be used as a soil fertilizer; inexpensive product available in convenient dosage forms
is a toxic chemical, which requires the use of protective equipment and precautions; restriction of use near bee farms; crops cannot be processed at high air temperatures; not suitable for spraying balcony and indoor plants
The described disadvantages relate more to the inconvenience of using the drug than to its effectiveness in combating diseases and pests. Therefore, you can count on the maximum benefits of colloidal sulfur when growing garden crops.
How to take sulfur and for what purpose?
Sulfur is mainly used externally as part of emulsions for acne, eczema and other skin diseases. However, taking it orally is also useful in some cases:
- metabolic disorders;
- sluggish functioning of the digestive tract;
- too oily skin, prone to excessive acne;
- inflammatory processes on the skin, psoriasis, eczema;
- decreased immunity;
- increased fatigue;
- weak, brittle nails and hair;
- diabetes, liver disease;
- allergies accompanied by skin rashes.
If you find yourself with a lack of sulfur, it can be replenished not only with pharmaceutical drugs. This trace element is found naturally in foods, especially of animal origin. Its quantity is high in meat and eggs. In plant products, it is found in cabbage, sprouted wheat, seeds, onions, and garlic. The presence of these products in the daily diet will help compensate for the deficiency of sulfur and other microelements.
The general approximate dosage for a person weighing about 70 kg is 0.25 g. The daily dose is divided by the number of meals and taken with food. For children, the dose is measured according to their weight and added to their food. Taking the drug in dry form, a child can inhale sulfur powder, which can lead to suffocation.
Food sulfur can be used either in its pure form or as part of tablets, dietary supplements, or in combination with nutritional yeast (in this form it is usually prescribed for acne). As part of the tablets, sulfur is easier to swallow and is better absorbed.
Products containing sulfur
Many organic products contain this useful element. Their daily use can bring benefits comparable to taking sulfur powder orally:
We recommend reading: The benefits and harms of leeks
- bulb onions;
- peas;
- a pineapple;
- zucchini;
- tomatoes;
- turnip;
- watermelon;
- nuts.
Recommended reading: Benefits of pineapple
Quail and chicken eggs contain the largest amount of the element.
There are also useful organic sulfur supplements on the market, such as chewable Siberian larch gum.
Instructions for use
When working with fungicides, it is important to follow the rules. Therefore, the instructions for using colloidal sulfur must be strictly followed.
Solution recipe
To obtain a working composition for plant protection, the procedure is divided into 2 stages:
- powder or sulfur granules are diluted with a small amount of water;
- Having mixed the mass until smooth, add liquid to the required volume (according to Table 1).
Before pouring the suspension into the sprayer, stir it for 5-7 minutes. The concentration in solutions is maintained taking into account the crops being treated and the diseases being combated.
Preparation and use of colloidal fungicide
| Disease | Cultures | Amount of sulfur per 10 liters of water (g) | How to apply |
| Powdery mildew | Greenhouse vegetables | 20 | Treat plants when infection is detected |
| Zucchini and cucumbers in open beds | 40 | ||
| Melons | 30 | ||
| Blackleg | Seedlings of cabbage, peppers, tomatoes | 40 | Water the holes 3 days before transplanting into open ground |
| Kila | Cabbage | ||
| Oidium | Grape | 30-60 | Treat the vine 3-4 times per season |
To protect fruit trees from most diseases, 80 g of suspension is used per bucket of water. For mites attacking plants, the concentration is higher - 100 g per 10 liters of water.
Sources
There are two types of sources:
| Vegetable: | Animal: |
|
|
What is sulfur and its role in the body
Sulfur, S (full name sulfur), is a macroelement assigned atomic number 16 in the periodic table.
This substance, known for its fetid odor in compounds and flammable properties, plays an important role in humans, being part of amino acids such as methonine, cystine, vitamins (for example, thiamine), hormones and enzymes (for example, insulin).
In the human body, the proportion of sulfur is 0.25% of the total mass.
Sulfur itself is not toxic, but compounds of the element with other chemical components, such as hydrogen sulfide, are poisonous.
It plays a key role in blood clotting. Its beneficial properties include protecting protoplasm from bacteria.
As part of the body's compounds, sulfur has benefits for the growth of hair, nails, skin, and also in protecting the body from aging.
Permissible doses
Information about the required daily intake of this macronutrient is quite contradictory.
The lack of accurate information is explained by the lack of a reasonable amount of data on the effect of the element on the body.
Experts agreed that for a person with no abnormalities in the body’s activity, 3-4 g of sulfur per day is enough.
With an optimal diet, rich in a variety of fruits and vegetables, meat and dairy products, additional consumption of sulfur is not required.
Some part of society should monitor the presence of this macronutrient in their diet, these are:
- Children;
- Persons with diseases of the musculoskeletal system;
- People actively involved in sports;
- Workers with heavy physical activity;

Purified sulfur – application, instructions
Purified sulfur is available in the form of ointments, pastes and powder:
- sulfuric ointment – from 10 to 50 g;
- sulfur-zinc-naphthalan paste – 40g;
- purified sulfur powder – 10 g;
Sulfur, purified indications
Purified sulfur is used:
- For pyrogenic therapy for: progressive paralysis; schizophrenia and others.
- In the treatment of skin diseases: seborrhea; psoriasis; scabies; sycosis.
- As an anthelmintic when infected with pinworms (enterobiasis).
Purified sulfur is also used to treat hemorrhoids, constipation, and so on.
Sulfur, purified instructions for use
Fully purified sulfur is used for various skin conditions and in 20%, 10% and 5% powders and ointments.
In addition, purified sulfur is used internally as a mild expectorant and laxative, 2.0; 1.0; 0.5 grams each.
For the treatment of enterobiasis, this drug is prescribed for consumption three times a day, but for 5 days, for adults - 1.0-0.8 g and for children - about 0.05 g, and for children aged 5 or 6 years - exactly 0.25 g.
After this, a four-day break is taken during the treatment process, during which enemas with the addition of sodium bicarbonate or sodium chloride are given every day at night. After the break, there is again a five-day cycle for taking sulfur, after which there is again a break of four days. This five-day cycle of sulfur treatment is carried out mainly from 3 to 5 times.
Application of purified sulfur internally and externally
It is worth noting that this drug does not work in dry form. In the presence of moisture, organic substances and alkalis, hydrogen sulfide, sulfur dioxide, various sulfur alkalis and oxygen are formed, which have a positive effect.
When used topically, the skin has an irritating, keratolytic effect (this is the process of formation of hydrogen sulfide and disulfides deep in the epidermis itself). It has a fairly weak but stable antimicrobial effect. In addition, the basis for a complete antiparasitic effect is the complete formation of hydrogen sulfide and anhydride.
After using this drug internally, sodium sulfite, sodium hydrosulfite and hydrogen sulfide are formed, which strongly irritate the receptors inside the intestines and act as a laxative.
To hydrogen sulfide, sulfur is able to be restored in the large intestine under the strict influence of bacteria and, of course, protein substances of the mucous membranes within an alkaline environment with the participation of glutathione and cysteine. About 10 percent of the sulfur introduced inside is restored, and the rest will be excreted along with the feces.
In addition, sulfur, precipitated in a finely dispersed state, is quickly reduced directly into hydrogen sulfide, then absorbed and after that may well cause poisoning in a person, since a laxative is not used. Sublimated purified sulfur is used as a laxative.
In addition, hydrogen sulfide can be partially absorbed from the intestines and, when released through the lungs, has an expectorant effect. Immediately after parenteral administration of this drug, sulfur causes a number of reactions in the body that are characteristic of nonspecific medical therapy by the appearance of irritation.
Because of this, the tone directly in the autonomic innervation significantly increases, then the protective forces in the human body are strengthened, the formation of a number of antibodies increases, and the ability of the human body to completely neutralize the poison also increases.
The needs of animals in the environment are met thanks to amino acids - L-methionine, L-cysteine and L-cystine, as well as heterocyclic compounds - thiamine and biotin.
Cystine and cysteine are found in the general composition of proteins, enzymes and certain hormones; These amino acids are also necessary to accelerate the rapid growth of animal fur, horns and hair.
In dermatology, it is used to treat eczema, scabies, furunculosis, dermatitis, trichophytosis and other lesions of the skin and body as ointments (10-30%), dusts and liniments.
This drug is prescribed as a mild laxative (but quite rarely), as well as an antidote for very acute chronic diseases with drugs, mercury, lead and other heavy metals, but at the same time all insoluble sulfur beneficial compounds with some heavy metal salts are formed inside the intestine.
Sulfur is also used to fully improve the metabolism of all substances, to accelerate growth, significantly enhance fermentation (bacterial) in the forestomach in ruminants, accelerate the growth of wool in sheep, hooves and horns in various animals.
Indications for use
Sulfur is used in folk practice and medicine, for example, in the field of dermatology: with the help of sulfur, doctors “fight” the symptoms of scabies, eczema and folliculitis. The mineral is used for seborrhea, psoriasis or hair loss. Doctors also prescribe it when diagnosing various skin acne, sycosis and herpes zoster.
In the presence of high pigmentation of the epidermis or the presence of freckles, sulfur can be used as an exfoliant, and in case of constipation - as a laxative. Very often, sulfur helps with chronic hemorrhoids. It is also prescribed as an anthelmintic.
When diagnosing polyarthritis or sciatica, the mineral is used as an irritant, and in the presence of progressive paralysis, body temperature is increased using a sulfur suspension. Sulfur is also used for severe poisoning with mercury, metals and hydrocyanic acid. At home, sulfur is used for inflammatory processes of the gums.
How to use sulfur
Sulfur-containing drugs are taken simultaneously with food.
You should only buy purified sulfur from the pharmacy.
Feed sulfur preparations for animals are not intended for human consumption.
The optimal course of treatment would be 1 month. If there is no expected benefit, you should stop taking it until you consult your doctor.
Important! Sulfur is used as prescribed by a doctor in the recommended dosage. Self-administration and prescription of the drug is not recommended
Sulfur powder for oral administration
Powder for internal use is prescribed for chronic diseases:
- polyarthritis;
- sciatica;
- haemorrhoids.
Sulfur for oral administration is purified and comes in tightly sealed jars. Taking purified sulfur is also prescribed for the treatment of enterobiasis.
Medical purified sulfur, or sulfuric anhydride, is beneficial in cases of:
- restoration of the body's defenses;
- for expectorant purposes: hydrogen sulfide is absorbed through the intestines and enters the lungs through the blood;
- for constipation, purified sublimated sulfur is prescribed;
- to enhance the body’s ability to neutralize weak poisons.
When taking sulfur, you can drink enterosorbents if you wish: they will reduce the harm of frequent gas formation that the drug can cause.
Sulfuric ointment
In addition to oral administration, medical purified sulfur is used externally in the form of ointments to treat:
- scabies;
- eczema;
- dermatitis;
- depriving;
- acne.
For dermatitis, medicinal sulfur ointment is recommended to be applied twice a day. The sulfur concentration in the preparation is 5%.
The sulfur concentration in acne ointment is 33%. It is applied to clean skin and dry skin. It is advisable to do the procedure in the evening and not rinse off immediately after applying the product.
During treatment of scabies, do not wash off the ointment from the skin. You should also not shower during therapy. Only after a 5-day course of treatment should you wash everything off thoroughly and put on clean clothes. Bed linen also needs to be changed.
Attention! Sulfur ointment smells unpleasant and leaves greasy marks
Total information
Location on the periodic table D.I. Mendeleev: in the old version - III period, III row, VI group, in the new version of the table - 16 group, 3rd period.
Physicochemical characteristics. Pure sulfur is yellow transparent and translucent crystals that can be easily crushed into a powder without much difficulty. In the presence of impurities, it can have orange, yellow-brown, brown and even black colors. Usually it contains additives of clay, sulfates, carbonates, bitumen and other substances. The shine is oily and greasy.
In nature it is presented in 2 forms - α-sulfur and β-sulfur. Alpha is characterized by a rhombic crystal appearance and a stable structure at temperatures up to 96 °C. When exposed to higher temperatures, alpha sulfur degenerates into beta sulfur, which already has monoclinic crystals and becomes resistant to higher temperatures. The density of the mineral is 2.05 -2.08 g/cm3.
Due to its hydrophobic properties, sulfur does not sink in water, although this mineral is heavier than H2O. However, when you add some alcohol or acetone, it sinks to the bottom. By the way, due to the fact that sulfur is found in many salts and acids, they are also poorly soluble in water. Sulfur is highly soluble in kerosene, turpentine, carbon disulfide and Canada balsam.
When heated, S gradually melts, transforming into a yellow mobile liquid, and with a further increase in the heating temperature to 160 ° C, the yellow color gives way to orange, then dark red, then, at 190 ° C, it thickens a little and after a while, at 300 ° C, it becomes again mobile.
Physicochemical characteristics
Sulfur is a soft, brittle mineral, insoluble in water and non-oxidizing acids. Natural sulfur crystals are sensitive to light and heat and should be stored in a dark, cool, dry place. Under normal conditions, sulfur is an odorless solid. There are several non-metallic modifications of sulfur.
Sulfur crystals show typical shapes of the orthorhombic system, such as pinacoids, prisms or bipyramids. Clear, intact, well-formed crystals in the shape of barrels or thick bars are very popular among collectors.
Orthorhombic sulfur is also called α-sulfur. When heated from 95.2 C, it turns into a yellow liquid melt, and λ-sulfur is obtained. If the mixture continues to be heated from 159 C, it gradually becomes viscous, forming μ-sulfur at 200 C. Above 250 C, the viscosity decreases, the boiling point is 444 C. If molten sulfur solidifies on the surface in large crucibles, long monoclinic crystalline needles are formed. This modification is called monoclinic sulfur or β-sulfur. When it cools below 115.2 C, it slowly turns back into rhombic sulfur. If the liquid melt is poured into a glass of cold water, elastic threads or a yellow-brown viscous mass called plastic sulfur is formed, which then gradually turns back into rhombic sulfur.
Sulfur is very reactive and forms chemical compounds with many elements. The exceptions are gold , platinum, iridium, nitrogen , tellurium, iodine and noble gases. In air, sulfur burns with a bluish flame, producing the toxic and pungent-smelling gas sulfur dioxide (SO2). When sulfur dioxide dissolves in water, it produces a solution of sulfur dioxide and, in small quantities, sulfuric acid .
The sulfur is attacked by oxidizing acids such as concentrated nitric acid . Other important sulfur compounds are sulfuric acid and its salts and sulfates.
- Alpha sulfur is the most stable form. It is also called rhombic sulfur. This modification forms lemon-yellow, rather brittle crystals.
- Beta sulfur is formed from alpha sulfur when heated to 95 C. Due to its crystalline form, this modification is also called “monoclinic” sulfur. Monoclinic sulfur has a light yellow color.
Sulfur becomes liquid at 113 C (orthorhombic) or at 119 C (monoclinic). At first the liquid turns yellow, and upon further heating it turns dark brown. If sulfur is heated to temperatures above 400 C, the viscous melt becomes liquid again. Liquid sulfur boils at 445 C. Sublimated sulfur is obtained by cooling sulfur vapor (light yellow powder). However, if the melt is rapidly cooled (quenching), plastic sulfur is formed.











