Calorie is the key concept of proper nutrition. However, if proteins, fats and carbohydrates are real substances, then calories (more precisely, kilocalories) refer only to units of measurement. They are used to calculate the nutritional value of the nutrients contained in food, as well as the body's overall energy needs.
Among other things, it is a mistake to talk about the absorption of calories - it is the nutrients that have a certain energy that are absorbed. Likewise, when playing sports (or during everyday activity), it is not calories that are burned at all, but carbohydrates, proteins or fats previously stored in the body.
What is the difference
Those who monitor body weight and count kcal do not really think about what this means. The reduction in calories is carried out actively, without particularly going into where it came from and where it went.
And most importantly, without thinking about it: calories and kilocalories - what is the difference between them:
- The calorie content of the product is always indicated on the packaging in kilocalories. If it says “N kcal”, you can’t believe it, all products have a calorie content, even the smallest one, and this sign is the ignorance of the manufacturer.
- To be clear, how many calories are in a kilocalorie? 1 kcal = 1000 calories.
- Exercise equipment in the gym often displays incorrect information about the calories burned on it. The measurement is made in kilocalories.
It must be remembered that products with zero or negative kcal do not exist in nature, no matter how their manufacturers claim in their advertisements.
Video
Why does the body need kcal?
Energy is required for normal human functioning. To move, breathe, circulate blood through the vessels, and even rest. Energy enters the body from the outside, with food. How much a person eats, so much energy will be generated.
How many kilocalories are contained in one gram of food components:
- carbohydrates – 4 kcal;
- proteins – 4 kcal;
- fats – 9 kcal.
Human food consists of these ingredients. Knowing the weight of the product in a dish, you can easily calculate the calories received from food.
The most popular breakfast is oatmeal. In 100 g there will be so many calories:
- Fat 6 g * 9 kcal = 54 kcal.
- Protein 12 g * 4 kcal = 48 kcal.
- Carbohydrates 51 g * 4 kcal = 204 kcal.
It turns out that 100 g of oatmeal contains 306 kcal. Metabolism will dispose of the received energy as follows: proteins will be converted into amino acids, carbohydrates into glucose and other simple sugars, and fats will be converted into glycerol and fatty acids required by our body.
Video
Calorie and its history
Lyubov Strelnikova “Chemistry and Life” No. 2, 2013
Artist E. Stanikova
Fat lands
Nowhere and never before have I seen so many huge, obese people as in the state of Texas several years ago. In the crowd on the streets of Austin, I felt like a dystrophic person.
Massive obesity in the United States has been a topic of constant debate in the press for more than a decade. However, this problem did not arise at the beginning of the 21st century. Half a century ago, in 1958, John Kenneth Galbraith, a renowned Harvard economist, first wrote in his best-selling book The Affluent Society that more Americans were dying from overeating rather than from malnutrition. He saw economic reasons in this. As Americans' basic needs for food, shelter, and clothing were satisfied by the mid-fifties, corporations began to invent and advertise new needs that they rushed to satisfy. The main thing is that they buy.
As a result, by the beginning of the 21st century, 61% of Americans already had health problems caused by excess weight. And the daily energy consumption of every person in the United States increased by almost two hundred kilocalories from 1977 to 1995, as Greg Kritzer writes in Fat Land: How Americans Became the Fattest People in the World"
, Boston, MA: Houghton Mifflin, 2003).
Obesity in the United States has become an epidemic. This is not just a metaphor: the World Health Organization also declares an “obesity pandemic.” And in the USA, the rate of its spread is the highest in the world: 13% of the population in 1962, 19.4% in 1997, 24.5% in 2004, 26.6% in 2007, 33.8% of adults and 17 % of children - in 2008, 35.7% of adults and 17% of children - in 2010.
Detailed statistics for Russia are not easy to find. It is often written about 15–16% of the adult population, but these figures probably date back to the early 2000s. In December 2012, the director of the Research Institute of Nutrition of the Russian Academy of Medical Sciences, chief nutritionist of the Ministry of Health of the Russian Federation V. A. Tutelyan said at a press conference that more than 25% of Russians are obese, and 50% are overweight. It seems that we are again trying our best to catch up with America...
Obesity kills 100,000 to 400,000 Americans every year and costs American society $117 billion. These costs are comparable to the costs of solving medical problems associated with smoking and alcoholism.
What's the matter? Is it just the overeating that Galbraith wrote about? Greg Kritzer in his book analyzes possible reasons, political, social and economic. For example, when food prices peaked in the 1970s, President Richard Nixon demanded action. As a result of the reforms of the Minister of Agriculture Earl Butz, restrictions on the import of cheap palm oil were lifted, and it was allowed to make sweet glucose-fructose syrup from corn using new technologies. These cheap, but high-calorie products began to be used in the manufacture of the vast majority of food products to make them accessible.
Fast food marketers also did not stand aside. They simply forced their customers to eat more by launching Big Macs and other super-sized meals. As a result, the calorie content of one meal at McDonald's increased from 200 kilocalories in 1960 to 610. And customers diligently devoured the bloated Superburgers - no one can resist the gift of food.
Finally, Kritzer describes the emergence of a “new culture without borders” that makes it easier and fashionable to consume all these fat-rich, nutrient-poor foods. If in earlier times preparing home-cooked dinners was a tradition, then in the 80s housewives stopped spending time on this: after all, you can go somewhere or order ready-made food at home. Meanwhile, popular books and TV programs promoted theories that the baby knew when he or she was full and when and what to eat. As a result, parents no longer have control over what and when their child eats, even if it's just French fries and hamburgers.
To somehow rectify the situation, the American government began to take measures, including the 1990 Nutrition Labeling and Education Act.
, NLEA), obliging manufacturers to write the calorie content of products and their composition on all packaging. And in 2008, New York became the first city where restaurant menus began to indicate the calorie content of dishes so that visitors could make an informed choice that would not cause harm to their health. Everyone once again started talking about calories and started counting them.
Calorie and calorimeter
Previously, any schoolchild knew what a calorie was: the amount of heat that is needed to heat one gram of water by one degree. The term "calorie" (from the Latin calor
- heat) was introduced into scientific use by the French chemist Nicolas Clément-Desormes (1779–1842).
His definition of the calorie as a unit of heat was first published in 1824 in Le Producteur
and appeared in French dictionaries in 1842. However, long before this term appeared, the first calorimeters—instruments for measuring heat—were designed. The first calorimeter was invented by the English chemist Joseph Black, and in 1759–1763 he used it to determine the heat capacities of various substances, the latent heat of melting of ice and the evaporation of water.
The famous French scientists Antoine Laurent Lavoisier (1743–1794) and Pierre Simon Laplace (1749–1827) took advantage of D. Black’s invention. In 1780, they began a series of calorimetric experiments that made it possible to measure thermal energy. This concept is found back in the 18th century in the works of the Swedish physicist Johann Karl Wilcke (1732–1796), who studied electrical, magnetic and thermal phenomena and thought about equivalents in which thermal energy could be measured.
The device, which later began to be called a calorimeter, was used by Lavoisier and Laplace to measure the amount of heat released in various physical, chemical and biological processes. At that time there were no accurate thermometers, so to measure heat it was necessary to resort to tricks. The first calorimeter was ice-cold. The inner hollow chamber, where an object emitting heat (for example, a mouse) was placed, was surrounded by a jacket filled with ice or snow. And the ice jacket, in turn, was surrounded by air so that the ice did not melt under the influence of external heat. Heat from the object inside the calorimeter heated and melted the ice. By weighing the meltwater that flowed from the jacket into a special vessel, the researchers determined the heat generated by the object.
A seemingly simple device allowed Lavoisier and Laplace to measure the heat of many chemical reactions: the combustion of coal, hydrogen, phosphorus, black powder. With these works they laid the foundations of thermochemistry and formulated its basic principle: “Any thermal changes that any material system experiences, changing its state, occur in the reverse order, when the system returns to its original state.” In other words, to decompose water into hydrogen and oxygen, it is necessary to expend the same amount of energy as is released when hydrogen reacts with oxygen to form water.
Also in 1780, Lavoisier placed a guinea pig in a calorimeter. The warmth from her breath melted the snow in his shirt. Then other experiments followed, which were of great importance for physiology. It was then that Lavoisier expressed the idea that the breathing of an animal is similar to the burning of a candle, due to which the necessary supply of heat is maintained in the body. He was also the first to connect the three most important functions of a living organism: respiration, nutrition and transpiration (evaporation of water). Apparently, since then they started talking about the fact that food burns in our body.
In the 19th century, thanks to the efforts of the famous French chemist Marcelin Berthelot (1827–1907), who published more than 200 works on thermochemistry, the accuracy of calorimetric methods greatly increased and more advanced instruments appeared - a water calorimeter and a sealed calorimetric bomb. The last device is especially interesting to us because it can measure the heat released during very fast reactions - combustion and explosion. A sample of the dry test substance is poured into a crucible, placed inside the bomb and the vessel is hermetically sealed. The substance is then ignited with an electric spark. It burns, giving off heat to the water in the surrounding water jacket. Thermometers allow you to accurately record changes in water temperature.
Apparently, in a similar calorimeter in the thirties of the 19th century, the famous German chemist Justus von Liebig (1803–1873) conducted his first experiments with food, who shared Lavoisier’s ideas that food is fuel for the body, like firewood for a stove. Moreover, Liebig named this firewood: proteins, fats and carbohydrates. He burned food samples in a calorimeter and measured the heat released. Based on the results of these experiments, Liebig, together with his colleague Julius von Mayer, compiled the world's first food calorie tables and, based on them, tried to calculate a scientifically based diet for Prussian soldiers.
A famous follower of Justus von Liebig was the American agricultural chemist Wilbur Olin Atwater (1844–1907). He was the first to think of measuring the energy content of food components and came up with a scheme for calculating the calorie content of any food product. He didn't have to start from scratch. Atwater spent three years (1869–1871) in Germany, where he studied the experience of European agricultural chemist colleagues. Here he was not only inspired by the ideas of physiological calorimetry sown by Liebig, but also mastered some experimental techniques.
Today he is called the father of nutrition. “Much of the knowledge we use today about food and its components comes from Atwater’s experiments,” says Erica Taylor, a chemistry professor at Wesleyan College in Connecticut, where W. O. Atwater once worked. Indeed, the values so familiar to us of the calorie content of carbohydrates (4 kcal/g), proteins (4 kcal/g) and fats (9 kcal/g) were first obtained experimentally by Atwater. But even now, one hundred and twenty years later, nutritionists use these figures when calculating the energy value of food. Atwater's system remains the basis for food labeling today. And in this sense, as one of the journalists correctly noted, Wilbur Atwater is the most quoted scientist in the world.
Atwater's Key Factors
As the American anthropologist Richard Wrangham writes in his book “Light the Fire: How Cooking Made Us Human” (Moscow, Astrel, 2012), Atwater dreamed of making it so that the poor could buy enough food with their modest means, providing themselves with the necessary energy. To do this, it was necessary to understand how many calories are contained in different foods and how many of them a person needs to provide energy for his life. At that time, our information about the composition of products was scanty. In the 70s of the 19th century, they did not yet know about vitamins, microelements, antioxidants and their importance for the body. The importance of calcium and phosphorus was recognized, but their role was not understood. However, Atwater was solving “energy” problems, and at that time they already knew for sure that three main components of food provide energy to the body: proteins, fats and carbohydrates. This is where Atwater needed a calorimeter bomb. In it, he measured how much heat is released during the complete combustion of an accurate sample of typical proteins, fats and carbohydrates. Of course, there are various proteins, as well as fats and carbohydrates. But their calorific value within each group did not differ much.
However, combustion heat alone is not enough. You need to know how much of each of these components is in your foods. The solution was found to be purely chemical. Using ether, Atwater extracted fat from a ground piece of food, the weight of which he knew exactly. And then he determined the weight of the substance (fat) that had passed into the ether. This way it was possible to calculate the lipid content in the product. By the way, this same simple method is still used today.
I had to tinker with proteins, since there is no analysis to determine the total amount of proteins in a particular product. However, Atwater knew that on average about 16% of the mass of protein is nitrogen. He figured out how to determine the amount of nitrogen in food, and through it he calculated the protein content.
There is a similar problem with carbohydrates: they did not know how to determine their total content in food. Arithmetic came to the rescue here. Atwater burned a sample of food and determined the amount of ash produced, which contained only inorganic substances. Now it was not difficult to determine the total organic content (the original weight of the food minus the ash). By subtracting the fat and protein mass from this value, Atwater arrived at the carbohydrate content.
However, not all the food we eat is absorbed by our body. How long does it idle? This was important to know and take into account when assessing the energy value of the product. To answer this question, Atwater had to examine the feces of people whose diets were precisely known. According to his calculations, it turned out that on average the share of undigested food is no more than 10%.
As a result of all these experiments and calculations, which took more than one year, Atwater finally proclaimed: the energy value of proteins and carbohydrates eaten by humans is 4 kcal/g, and fats - 9 kcal/g. These magic numbers were called the Atwater Factors, and his approach was called the Atwater System. By 1896, he had developed calorie tables. They were the ones used by the compilers of the US Department of Agriculture National Nutrient Database reference book and the Food Composition reference book.
Atwater's system turned out to be extremely versatile and tenacious. Suffice it to say that the general factors remain unchanged to this day. But at the same time, the system is flexible and open to various additions and clarifications. Atwater himself eventually added alcohol (7 kcal/g) to his regimen, rightly considering it a high-calorie source of energy. True, after the scientist published the results of the study, alcohol producers immediately seized on the thesis “alcohol provides a lot of calories to the human body” and began to actively use it in advertising their products. This greatly upset Atwater, and he considered it necessary to give students one lecture every year on the dangers of alcohol and the benefits of moderation in everything.
In the twentieth century, nutritional biochemistry developed extremely actively, allowing researchers to obtain more and more new data. Already in the second half of the last century, new factors for dietary fiber (non-starchy polysaccharides) were introduced into the system. It is known that this group of substances is absorbed much worse than carbohydrates, so their energy value was noticeably lower - 2 kcal/g. It was even possible to take into account the energy that the body expends to produce urine and gases.
In 1955, general factors were supplemented with specific ones: egg white - 4.36 kcal/g, brown rice protein - 3.41 kcal/g, etc. The same is with the nitrogen content in protein: instead of the average value of 16%, they began to use specific numbers - for example, 17.54% for pasta protein and 15.67% for milk protein.
However, the effect of all these small clarifications turned out to be so small that many nutritionists still use Atwater’s general factors. Much more serious problems with this system are related to something else.
Unaccounted factors
The first major flaw is that the Atwater system does not take into account the energy expenditure of digestion. Humans, of course, spend significantly less energy on digestion than, say, snakes and fish. But nevertheless, these expenses are noticeable. We have to pay with energy to digest food. Fat is easiest to digest, then carbohydrates, and proteins are the worst. The higher the proportion of protein in food, the higher the costs of digestion. Wrangham, in his book, mentions one 1987 study that found "that people whose diets were high in fat gained the same weight as those who ate nearly five times as many calories as carbohydrates." However, not only the chemical composition of the product is important, but also its physical state. Obviously, the body will spend more energy on digesting raw food rather than cooked food, hard rather than soft, consisting of large particles rather than small ones, cold rather than hot. It turns out that the calorie content of food that has been repeatedly processed, chopped, steamed, boiled and maximally softened is higher than that of food prepared from the same products, but processed less intensively.
When we go to the hospital to visit a sick friend or relative, we bring with us chicken broth and boiled chicken breast, or steamed cutlets, or mashed potatoes... Not because it’s tasty and easy to prepare (someone doesn’t like chicken breasts). But because this is the most tender meat of chicken, where there is practically no connective tissue. It is very soft, so it is easily digestible, without taking away excess energy from the patient for digestion (it will be useful to him for recovery) and at the same time giving more calories. In this sense, the calorie content of chicken breasts is higher than that of chicken legs.
A good illustration of this is a well-known study carried out by Japanese scientist Kyoko Oka et al., “ Food texture Differences Affect Energy Metabolism in Rats
", "Journal of Dental Research", 2003, 82, 491–494). The researchers kept 20 rats on different diets: half were given regular pellet food, which had to work hard to chew, and the other half were fed the same pellets, only puffed up like breakfast cereal. The conditions of keeping the animals and their load were the same. It would seem, how can the method of cooking affect the growth of animals? How could it?
The rats switched to a diet containing different pellets at four weeks of age. At week 22, the differences became noticeable to the naked eye. Rats fed the soft diet weighed an average of 37 grams (about 6%) more than those fed the hard kibble, and had an average of 30% more fat, which is classified as obese. The rats got fatter from soft, highly processed food because they spent significantly less energy on digestion. It turns out that air flakes have more calories than solid granules.
The physical state of food is a trap for the Atwater system. He believed, and this is built into his system as one of the main factors, that the body does not digest 10% of the food that is excreted in feces. Atwater thought that this value was constant and did not depend on the consistency of the food. Perhaps in his time there was no incredibly finely ground snow-white flour. But today we know that this flour is 100% digestible. And if we eat baked goods made from coarse flour, then a third of it is excreted from the body undigested.
The Atwater system has another pitfall, which can be called “biodiversity.” We are all very different, different genetically, and therefore biochemically and metabolically. How many times have we been surprised by the voracious appetite of thin people who, despite large volumes of food consumed, do not gain weight. The fact is that thin people normally spend more energy on digestion than fat people. Therefore, after eating food of the same calorie content, an overweight person will gain more weight than a thin person.
So, Atwater’s system does not take into account the significant contribution that its physical properties and methods of preparation make to the calorie content of food, and finally, the metabolic portrait of each of us. This means that we cannot use this system to assess the real nutritional value of our own diet. There are more and more high-calorie products on the shelves, although they do not look like them, judging by the composition and declared calorie content on the labels. They mislead us because none of what we talked about in this chapter is taken into account in these inscriptions. Meanwhile, we continue to gain weight from food that is easy to digest.
Can all these additional, but so important factors be taken into account in Atwater's system? Extremely difficult, if not impossible. Methodologically, this is an incredibly difficult task. After all, it will be necessary to carry out a huge number of experiments in order to obtain real nutritional value of specific products, taking into account their consistency, method of preparation, combination with other products and our biochemical individuality.
Can we do without calories?
How many calories does a person need? To this question, which Atwater posed to himself at the very beginning of his research, he was able to give a comprehensive answer. Together with his colleagues at Wesleyan College Edward Rosa and Francis Benedict, he designed a special ventilated calorimeter chamber in which a person could stay, work and rest. The heat it generated was determined by the difference in temperature of the water that flowed through a system of tubes laid in the chamber - at the inlet and outlet. With its help, in 1896, he began to study how much energy a person spends at rest, wakefulness and during various activities, how much oxygen he consumes and how much carbon dioxide he produces. The objects of the study were primarily his students.
Based on the results of these measurements, Atwater was the first to calculate the balance between the energy entering the body with food and consumed by a person. He confirmed that the law of conservation of energy also works in the human body: it does not disappear anywhere, but passes from one form to another. It is interesting that before Atwater, there was an opinion in scientific circles that the first law of thermodynamics applied to animals, but not to humans, since humans are unique. Atwater not only refuted this misconception, but also proved for the first time: if a person does not fully use the energy that enters his body with food, then it is stored in the form of excess kilograms.
Atwater studied the diets of a huge number of different families from different walks of life. Analyzing the results, he sadly noted that people are eating more and more fatty and sweet foods and moving less and less. Even then, he talked about the importance of a cheap and effective diet that included more proteins, beans and vegetables instead of carbohydrates.
Atwater made enormous contributions to nutritional science. These are not only the results of more than 500 scientific papers and one and a half hundred articles. He managed to achieve the creation of the US Federal Food Research Foundation. In 1894, for the first time in a bill, the US government appropriated ten thousand dollars for food and diet research. Atwater did most of them. One hundred years later, federal support for these programs has increased to $82 million. And he foresaw that we would begin to get fat because we were eating more and moving less. Foresaw at the end of the 19th century.
Caloric content and chemical composition are still calculated using the Atwater system, albeit modified in the 20th century. Yes, today we understand that she gives rough estimates. But it's better than nothing.
Apparently, meticulous calorie counting in stores and restaurants is losing its meaning. What to focus on? To simple rules that have stood the test of time and do not need adjustment: eat in moderation, move more, avoid fast food and sugary drinks, eat more vegetables and fruits, cook your own homemade food from fresh ingredients. You know all this as well as I do.
But here is another argument worthy of attention. Judy McBride of the USDA Agricultural Research Service put it very well: “Who knows how many unknown components we have not yet discovered or noticed in foods that are beneficial and necessary for our bodies? That’s why it’s so important to get your nutrients from fresh, whole foods rather than from vitamin supplements.”
Finally, I offer you a few rules (64 in total), taken from the book of the popular American journalist Michael Pollan, “The Nutrition Bible,” which was published by the Astrel publishing house last year.
- Rule 1: Eat real food, not manufactured stuff.
- Rule 8: Avoid foods that are advertised as healthy.
- Rule 13. Eat only what will spoil later.
- Rule 20: Anything shoved through your car window is not considered food.
- Rule 27: Eat animals that have been well fed themselves.
- Rule 29: Eat like an omnivore.
- Rule 37. The whiter the bread, the faster the death.
- Rule 39. Eat anything if you prepared it yourself.
- Rule 42: Be skeptical about non-traditional dishes.
- Rule 44: Pay more, eat less.
- Rule 47. Eat out of hunger, not out of boredom.
- Rule 49: Eat more slowly.
- Rule 52. Buy small dishes.
- Rule 56: Snack only on unprocessed plant foods.
- Rule 57. Do not refuel in the same place as the cars.
- Rule 58. Eat only at the table.
- Rule 59. Try not to eat alone.
- Rule 63. Cook yourself.
- Rule 64: Break the rules from time to time.
Where do they go then?
The body processes complex components of foods into simple ones, and then they go:
- Amino acids - from them muscle tissue, some hormones and enzymes are formed.
- Glucose is used to nourish cells. Its excess is deposited in “storage areas”: muscle and liver cells.
- Fats are used as fuel. Some of them go to the liver and are processed into cholesterol. When too much of it is received, it is deposited in the subcutaneous layer. These are exactly the fats that all females and a sufficient number of the stronger sex struggle with.
How is caloric intake distributed if you need to gain weight:
- Proteins – 30% of the total diet.
- Fats – 35% of food.
- Carbohydrates – 45% of the diet.
If you want to lose weight, then the percentage component changes:
- Proteins – 30% of the total products.
- Fats – 20% of products.
- Carbohydrates – 50%.
This is important: The amount of kcal for a person’s daily requirement should be calculated individually. As a result, the amount of proteins, fats and carbohydrates will change significantly.
How to calculate the calorie content of foods
Quite often, young girls try to lose weight by reducing calories. At the same time, they maintain their usual diet, just monitor the balance.
Video
The important principles in this method of losing weight remain:
- The amount of kilocalories eaten per day should be equal to or less than the amount of energy consumed during the day.
- It does not matter what products are used. It is important not to overstep the target limit and not to eat too much.
- You cannot significantly reduce the daily kcal norm so as not to provoke a malfunction of the systems and not get any diseases.
The calorie content of foods is calculated as shown in the example of oatmeal:
- Ingredients for preparing porridge: 200 g of cereal, a liter of milk, 2 tablespoons of sand, a pinch of salt, a spoon of cow butter.
- Calorie content: cereal – 732 kcal, milk – 640 kcal, sand – 199 kcal, butter – 149.6 kcal, salt – 0 kcal.
- The final result of the resulting porridge is 1720.6 kcal.
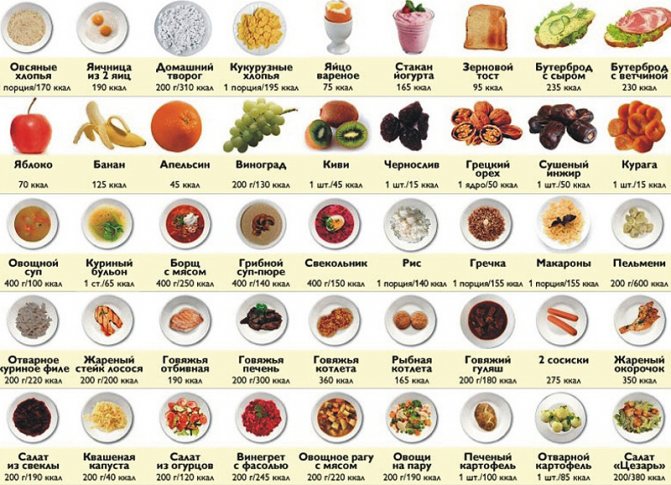
The numerical index of the calorie content of a product is available in a table that can be found on the Internet or in specialized literature.
When calculating, you need to take into account some nuances:
- The indicator for boiled cereals and pasta is three times less than the same product in its raw form.
- The weight of boiled meat decreases quite significantly, but rice, on the contrary, becomes larger.
- Dried foods (fruits, mushrooms, berries and crackers) have a much higher calorie content than the same foods in their original form. Here you need to calculate in this way: first, calculate the indicator x - the difference in the weight of the dried product, and then multiply the indicator from the table by x.
- In soup, you need to calculate not only those products that are included in the broth, but also seasonings, sour cream and everything that is usually added directly to the plate.
Nutrition must be monitored carefully, taking maximum care to ensure that healthy foods enter the body.
How many calories should you consume per day?
The answer to this question depends on many factors: age, height, current weight, activity level and metabolic health. To lose weight, a general rule of thumb is to consume 500 fewer calories than your body needs to maintain your current weight. This will help you lose about 0.45 kg of body weight per week. Below are the average calorie values that Health.gov takes into account for these factors. Appendix 2. Estimated Calorie Needs per Day, by Age, Sex, and Physical Activity Level – Published: 2002.
Women
- The average moderately active woman between the ages of 26 and 50 should eat about 2,000 calories per day to maintain her weight and 1,500 calories per day to lose about 0.45 kg of weight per week.
- Women who are active and walk more than 4 km per day should consume 2,200 calories or more per day to maintain their weight and at least 1,700 calories to lose 0.45 kg of weight per week.
- Young women in their 20s have higher calorie needs. They require about 2,200 calories per day to maintain weight.
- The average moderately active woman over 50 years of age requires about 1,800 calories per day to maintain weight and 1,300 calories per day to lose weight, which equates to 0.45 kg per week.
These estimates do not apply to pregnant or breastfeeding women because they have significantly higher caloric needs.
Men
- The average moderately active man aged 26-45 needs 2,600 calories per day to maintain weight, and 2,100 calories per day to lose 0.45 kg per week.
- Active men who walk more than 4 km per day may need 2800-3000 calories per day to maintain their weight and 2300-2500 calories per day to lose 0.45 kg of weight per week.
- Young men aged 19-25 need an average of 2,800 calories per day to maintain weight and up to 3,000 if they are active. To lose 0.45 kg per week, moderately active young people should consume 2300-2500 calories per day.
- Between the ages of 46 and 65, moderately active men need an average of 2,400 calories per day.
- After age 66, the average calorie requirement for men drops to 2,200 calories per day.
Children
Children's caloric needs vary greatly depending on their age, size and activity level.
While the average toddler needs 1,200-1,400 calories per day, the average moderately active teen needs 2,000-2,800 calories per day. Active teenagers need even more. Children who grow and develop normally and exercise regularly do not usually need to count calories. When they have access to healthy foods, most active children will naturally eat the amount of food their bodies need.
How to calculate your daily caloric intake
To determine calorie content, you need to take into account some indicators:
- Metabolism (OM). It is affected by work schedule, physical activity, and nutrition. OB is calculated as follows: weight multiplied by 20 kcal.
- Age. How to calculate: for every 10 years after age 20, reduce by 2%.
- Floor. Typically, males require more calories.
- Rhythm of life. The percentage of activity is calculated: inactive - 20%, sedentary with little activity (going to the store, cleaning, walking, etc.) - 30%, average - 40%, high (sports, physical work) - 50%.
- Physical activity (PA) is calculated as follows: OB needs to be multiplied by the percentage of the rhythm of life.
- Percentage of energy during food digestion (PEPP). It is determined as follows: (FA is summed with OB) and multiplied by 10%.
Video
The daily intake of kcal is equal to – PEPP + FA + OB. The final indicator must be adjusted to your age; this is to subtract 2% from the result for the 10 years after the 20-year mark. If the total consumption rate is calculated for weight loss, then the result should be clarified: the daily calorie rate from which is subtracted (weight multiplied by 7 kcal).
Please note: It is necessary to take into account the energy needs of each person individually. Children and adults will have different meanings. Athletes and pregnant women should increase their calorie intake.
Low and high calorie foods
Which foods have many or few calories? This is affected by the chemical content. The lowest calorie foods are those whose energy value is less than 40 kcal per 100 g.
Which foods are low in calories:
- Vegetable products: cucumbers, lettuce, greens, garlic, sweet peppers, any onions, beets.
- Fruits and berries: citrus fruits, blackberries, strawberries, quince, cherries, pineapple, cherry plum, lingonberries, kiwi, apple, pomegranates and raspberries.
- Meat products: chicken, rabbit, lean beef, kidneys.
- Fish products: pollock, hake, blue whiting, flounder, shrimp, smelt and other types of river fish.
- Dairy products: all, only low fat.
- From sweets: marshmallows and marshmallows, marmalade.
- From flour - rye bread.
Those foods that have a high calorie content are those that have from 500 to 900 kcal per 100 g. Eating them is unsafe for your figure.
Video
These products include:
- Any types of oils.
- Pork is fatty, lard.
- Raw smoked sausages.
- Cakes with cream.
- Nuts of any kind.
- Milk chocolate.
High-calorie foods include alcoholic drinks, and they also cause increased appetite.
What are empty calories?
Empty calories contain little or no nutrients. They often come from added sugars and solid fats. Solid fats are fats that solidify at room temperature, such as butter and the fats found in some meats. They can occur naturally, but are often added to food.
Many fast foods contain empty calories. Ice cream, soda, cheese, pizza and meat products such as hot dogs and sausages are examples of popular foods high in empty calories.
Some of these foods, such as cheese and pizza, also contain nutrients (cheese is high in calcium and contains protein; pizza sauces, toppings, and crusts may contain nutrients), but other foods, such as soda and most candies, contain only empty calories.
Expert opinion
Smirnov Viktor Petrovich Dietitian, Samara
Many healthy eating enthusiasts do not know what calories are. How much energy does a person spend per day? Let's give a simple example. The article provides a table of a one-day menu for a person with a light level of physical activity. It is approximately 2500 kilocalories per day. As you know, one calorie heats 1 gram, or 1 ml, of water by 1 degree. What can 2500 kilocalories, or 2.5 million calories, do? First of all, such an amount of energy, however, purely theoretically, will allow heating 1 gram of water by 2.5 million degrees, and this is the temperature of thermonuclear fusion, which occurs in the depths of the sun and is the source of all the energy received by the Earth.
And here is the second example, more “down to earth”. Two large household buckets occupy a total volume of 24 liters (12 liters each). What can the same daily flow do to this volume of water? Simple calculation: (2500000/24000 ml). As a result, the balance is 104. Thus, the amount of energy consumed per day by a person at a “sedentary” job can boil two full buckets of water. Therefore, a person on a low-calorie diet and trying to lose weight should not complain that he receives very little energy. It is enough to remember these numbers, and it will immediately feel easier for him.
How are these terms used?
The regulations require food and drink manufacturers to place nutrition labels on their products. It must state, among other information, the amount of energy the item contains per serving or NIH weight. Nutrition Labeling: Issues and Directions for the 1990s.
A nutrition facts label helps inform the consumer about the healthfulness of packaged foods and drinks and whether they contain ingredients that you may need to avoid due to allergies, intolerances or personal preference.
Depending on where you live, this label may express the energy value of the food or drink in calories, kcal, kJ, or a combination of these. Below is a list of countries and the symbols they use to refer to energy:
- Russia: kJ (kJ) and kcal (kkal)
- US: calories (kal)
- Canada: calories (kal)
- European Union (EU): kilojoule (kJ) and kcal (kkal)
- Australia and New Zealand: kJ (kJ) or both kJ (kJ) and kcal (kcal)
- China: kJ (kJ)
Manufacturers determine the number of calories a food or drink contains based on the amount of energy-providing nutrients it contains. The 3 main nutrients that provide energy are protein, carbohydrates and fats. Protein and carbohydrates provide about 4 calories (16.7 kJ) per gram and fat provides 9 calories per gram (37.6 kJ) NIH. Diet and Health: Implications for Reducing Chronic Disease Risk.
Alcohol also contains 7 calories (29.3 kJ) per gram.
Manufacturers round to the nearest 1 gram, so if you measure calories or kJ, they may add up slightly different from the number on the label. Moreover, food labels containing fiber, which is classified as a carbohydrate, may contain fewer calories than you calculated. This is because fiber, depending on the type, is either indigestible or poorly absorbed, resulting in zero or few FDA calories. Guidance for Industry: The Declaration of Certain Isolated or Synthetic Non-Digestible Carbohydrates as Dietary Fiber on Nutrition and Supplement Facts Labels – Published: June 2018.











